chem y10 mid years
1/146
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
147 Terms
what side are reactants in a chemical reaction
left hand side
what side are products in a chemical reaction
right hand side
what does the state symbol ‘(s)’ mean
solid
what does the state symbol ‘(l)’ mean
liquid
what does the state symbol ‘(g)’ mean
gas
what does the state symbol ‘(aq)’ mean
aqueous
formula of water
H2O
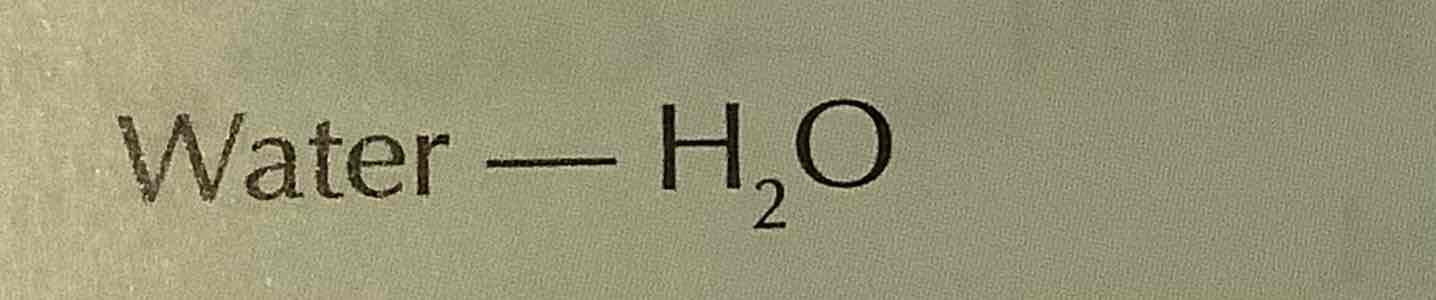
formula of ammonia
NH3
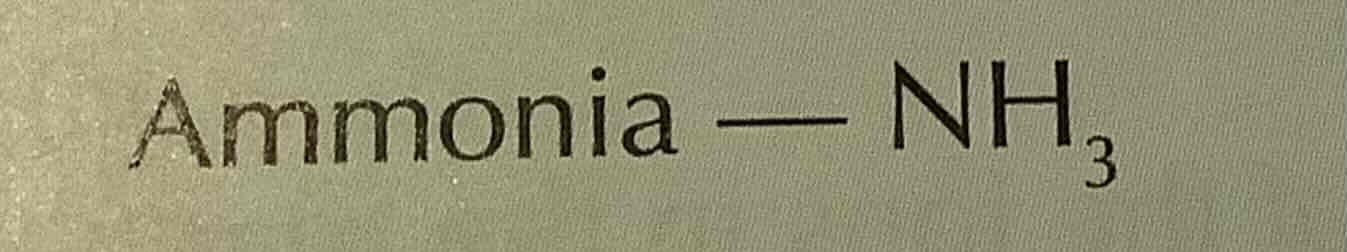
formula of carbon dioxide
CO2

formula of chlorine
Cl2
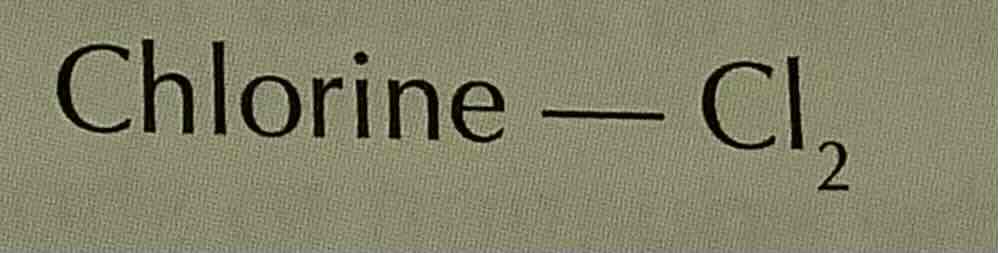
formula of ammonium (ion)
NH4+
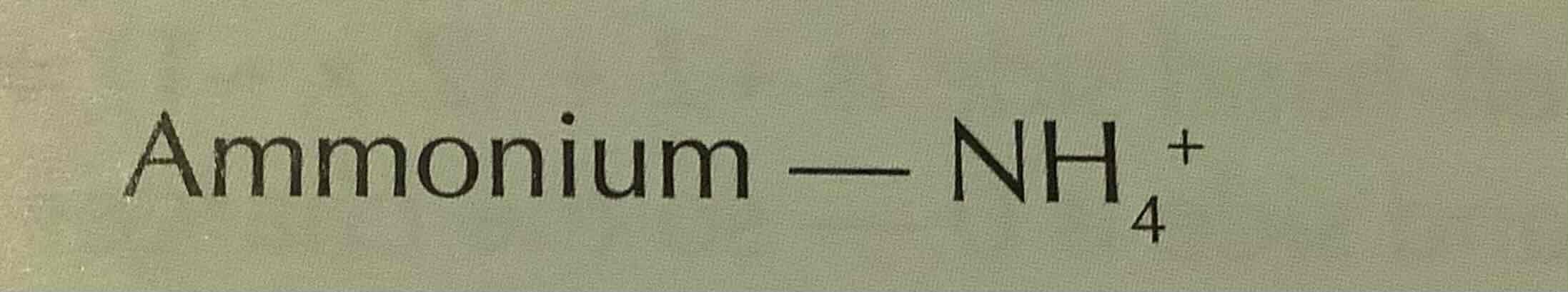
formula of hydroxide (ion)
OH-

formula of nitrate (ion)
NO3-
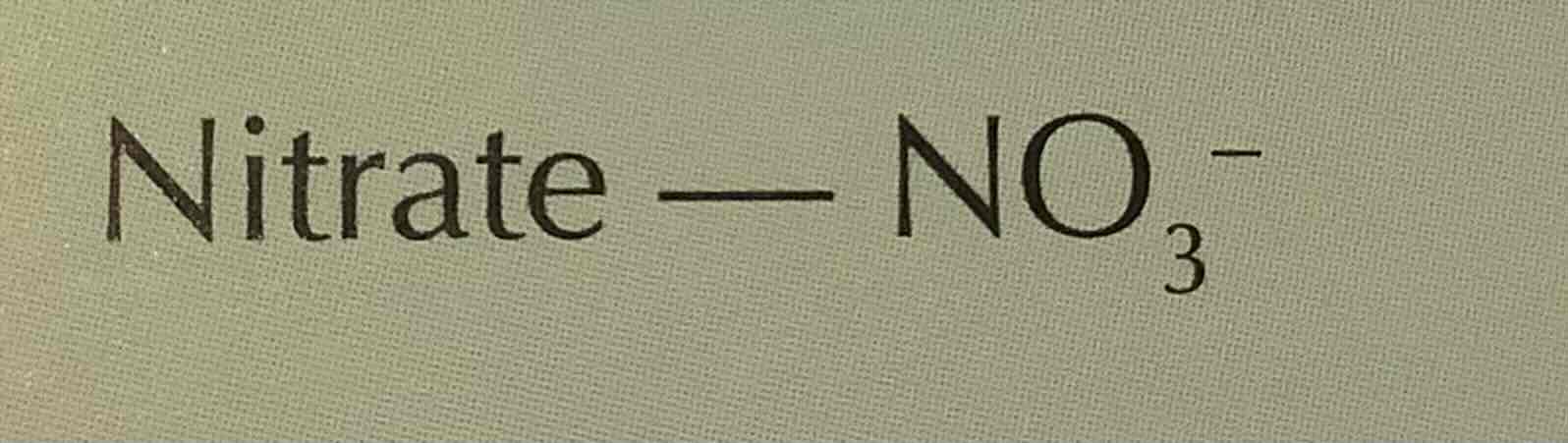
formula of carbonate (ion)
CO3 2-

formula of sulfate (ion)
SO4 2-
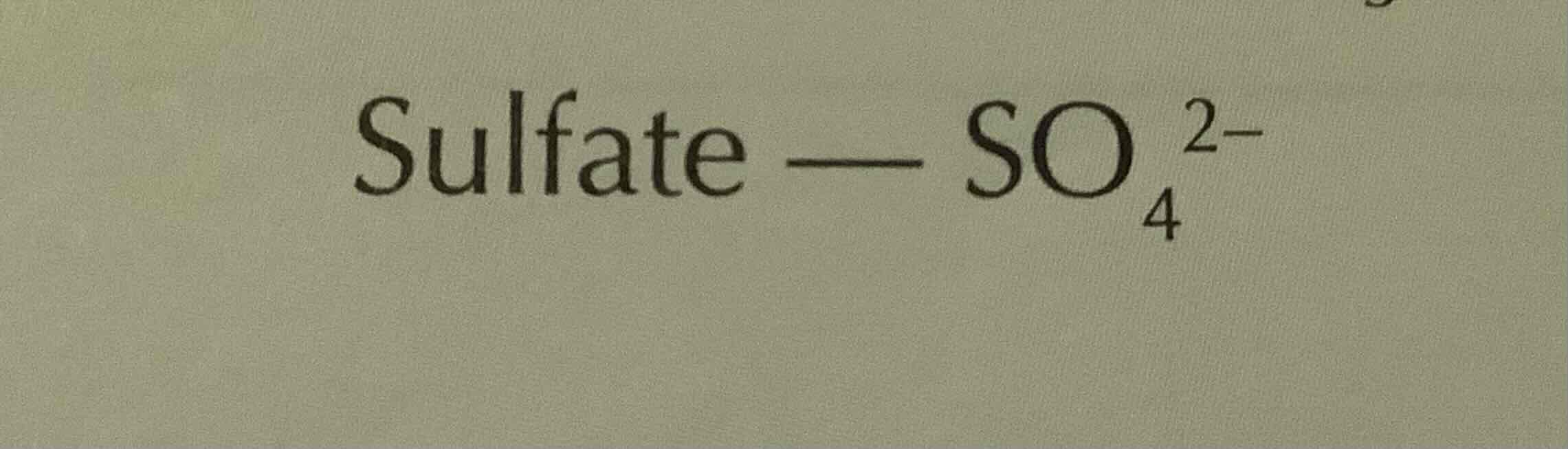
hazard meaning
something that could potentially cause harm
risk meaning
the chance that a hazard will cause harm
the plum pudding model
An early model which was based on a scientific idea on atomic structure was the plum pudding model. In this model, it was believed the atom was a sphere of positive charge with negatively charged electrons scattered around the atom like plums in a pudding
relative charge of protons
+1
relative charge of neutrons
0
relative charge of electrons
-1
relative mass of protons
1
relative mass of neutrons
1
relative mass of electrons
0.0005
radius of atom
1 × 10-10m
what does the atomic number tell you
the number of protons in an atom
what does the mass number of an atom tell you
the total number of protons and neutrons in the nucleus
what is an isotope
atoms with the same number of protons but a different number of neutrons
why are some rfms of elements not whole numbers
the rfm is the average mass of all the isotopes of the element, so sometimes they aren’t whole numbers
how did mendeleev organise the periodic table
in order of increasing atomic mass
how is the modern period table organised
in order of atomic number
vertical columns in the periodic table are called..
groups
horizontal rows in the periodic table are called..
periods
as you go down a group in the periodic table, the ___ increases
number of valence shells
electronic configurations arranged..
2, 8, 8, etc
what is an ion
an atom with a positive or negative charge due to losing or gaining electrons
negative ion
anions
positive ions
cation
ions ending with -ate are..
negative ions containing oxygen and at least one other element
ions ending with -ide are..
negative ions containing only one element (apart from hydroxide ions which are OH-)
ionic bonding
transfer of electrons between atoms to form ions
how are ions in ionic compounds held together
a giant lattice by electrostatic forces
why do ionic compounds have high melting points
a lot of energy is needed to overcome the string electrostatic forced that hold the ions together
why can’t solid ionic compounds conduct electricity
the ions are held in fixed positions
why can melted or dissolved ionic compounds conduct electricity
the ions are free to move
how do simple molecules form
when atoms join together through covalent bonds
ionic bond
a strong electrostatic force of attraction between these oppositely charged ions
covalent bond
a shared pair of electrons between two atoms
why do simple molecular substances have low melting points
the forces between molecules are weak so not much energy is needed to overcome them
what are giant covalent structures
structures made up of lots of atoms that are all bonded to each other by strong covalent bonds
why do giant covalent structures have high melting points
lots of energy is needed to break all the strong covalent bonds that hold atoms together
metal structure
the atoms in metals are arranged in a regular pattern, they consist of giant structures because they have lots of atoms
why do metals have high melting points
large amounts of energy are needed to break the strong electrostatic forces between the metal atoms and the delocalised electrons
why are metals malleable
because in a metal the atoms are arranged in layers that can slide over each other
how do metals conduct electricity and heat
the electrons in metals are delocalised, so they aren’t bound to one atom, and are free to move around
how to calculate rfm
all the relative atomic masses of the atoms in that compound added together
empirical formula
the simplest possible whole number ratio of each element in that compound
molecular formula
the actual number of atoms of each element within the compound
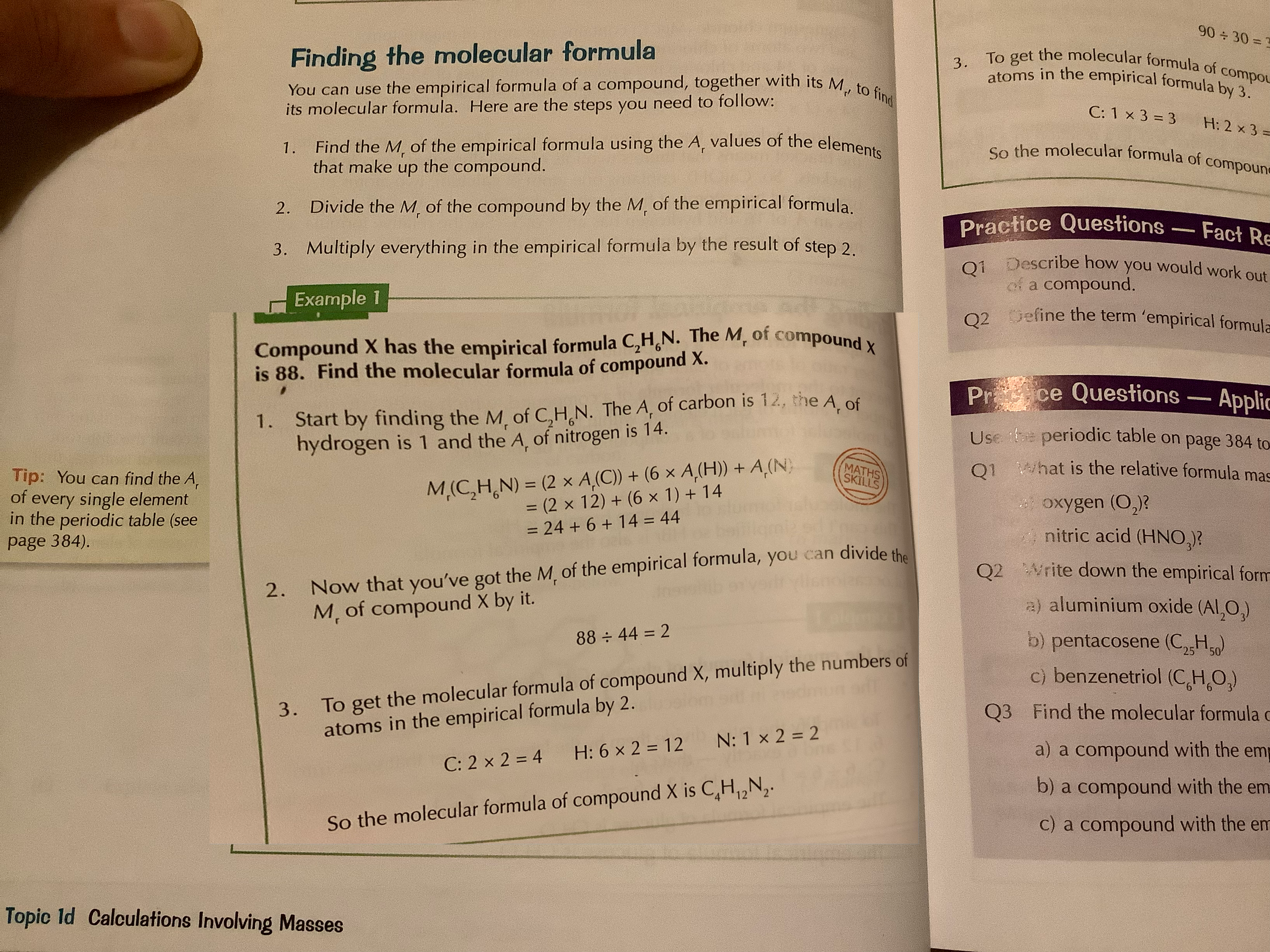
how to find the molecular formula
see picture
conservation of mass
during a chemical reactions no atoms are destroyed and no atoms are created, this means there are the same number and types of atoms on each side of a reaction equation
calculating empirical formula from masses
see image

what is concentration
the amount of substance in a certain volume of a solution
formula of concentration
concentration (g dm-3) = mass of solute (g)
volume of solution (dm3)
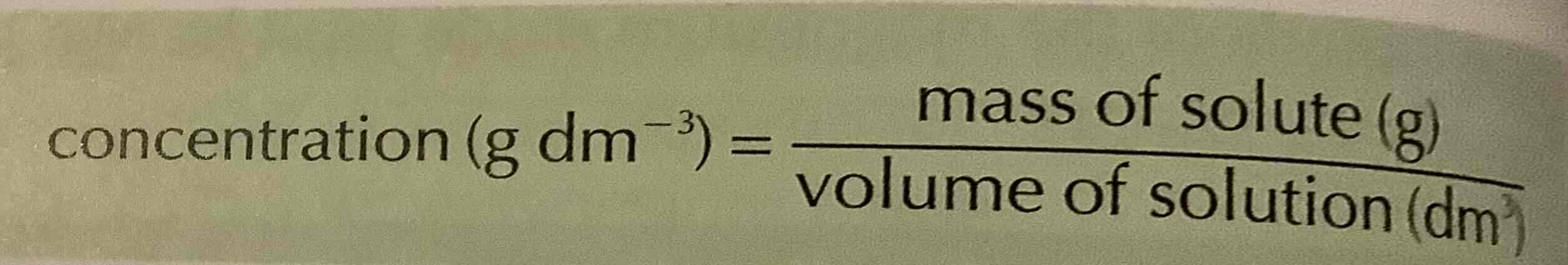
calculating the mass of a product
see image

calculating the mass of a reactant
see image

limiting reactants
the reactant that is used up first (because it limits the amount of product that is formed), any other reactants are in excess
avogadro’s constant
6.02 × 10²³
what is a mole
one mole of any substance is the amount of that substance that contains an avogadro’s number of particles, so 6.02 × 10²³ particles (the particles could be atoms, molecules, or ions)
the mass of one mole of atoms or molecules of any substance is exactly the same number of…
grams as the rfm of the element or compound

how to work out the moles of a substance in a certain number of particles
see image

formula for moles
moles = mass
mr

how to calculate how many atoms there are in a given mass of substance
see image

balancing equations using reacting masses
see image

in solids..
strong forces of attraction between particles, the forces hold the particles close together in fixed positions to form a very regular lattice arrangement. the particles in a solid don’t have much energy so they don’t move from their positions, because of this all solids keep a definite shape and volume. the particles vibrate about their positions and as the temp increases the particles vibrate more.
in liquids..
there are weak forces of attraction between the particles. the particles are arranged randomly and are free to move past each other, but stick close together. they have a definite volume but don’t keep a definite shape. the particles in a liquid are constantly moving and the liquid gets the faster the particles move
in gases..
the forces of attraction between particles are very weak. the gas particles are free to move constantly and randomly, travelling in straight lines until they collide with another particle or the walls of the container. the particles are very far apart, so much that most of a gas is empty space. don’t have a set shape and fill the shape of the container. the hotter a gas gets the faster the particles move and causes the particles to hit the walls of the container more frequently causing pressure to increase.
state change from solid to liquid
melting
state change from liquid to gas
boiling
state change from gas to liquid
condensing
state change from liquid to solid
freezing
state change from solid to gas
sublimation
state change from gas to solid
deposition
physical change
a change in the arrangement or energy of the particles, not the particles themselves. can be undone by heating or cooling the substance
chemical changes
happen during chemical reactions, when bonds between atoms break and atoms change places. so atoms are rearranged to form different substances. unlike physics changes chemical changes are difficult to reverse
a pure substance
a substance completely made up of a single element or compound
a mixture
a substance containing more than one compound or different elements that aren’t all part of a single compound
how to test for purity
if the substance is a mixture then it will melt gradually over a range of temperatures, e.g. pure ice melts at 0°C and pure water boils at 100°C, there’s no range

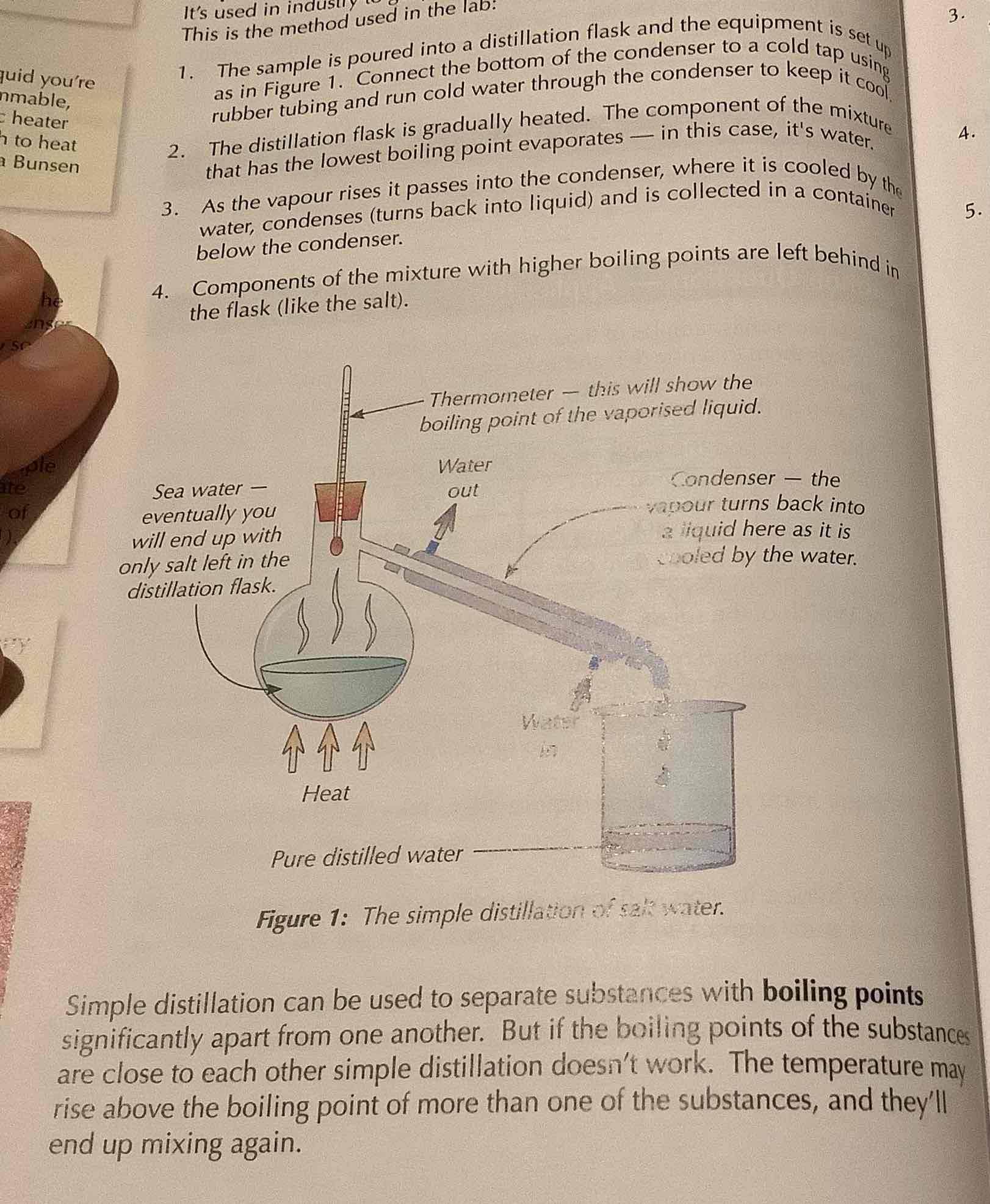
simple distillation
used to separate out a liquid from a mixture, eg used in the industry to get pure water from sea water
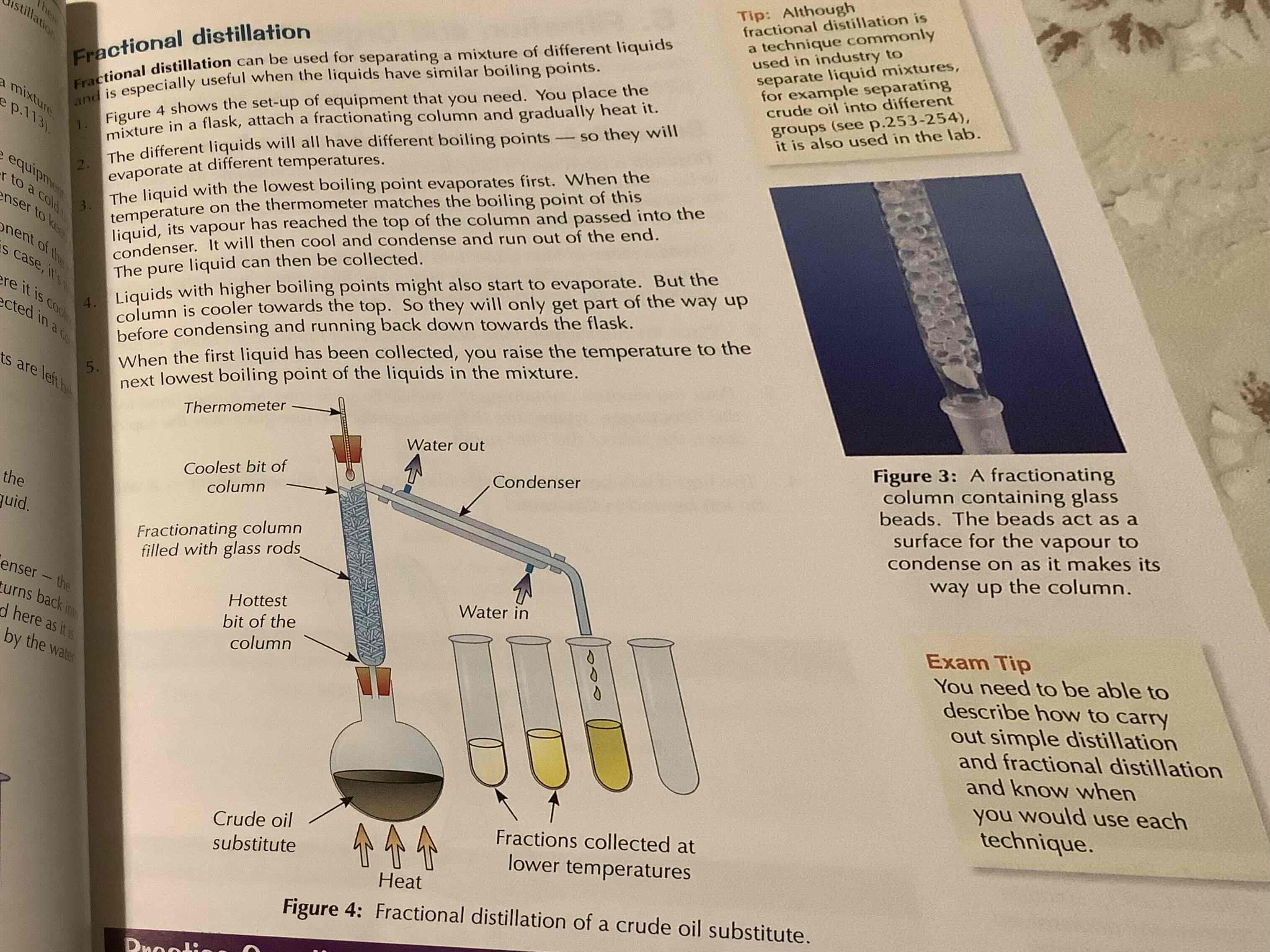
fractional distillation
see image
filtration
see image

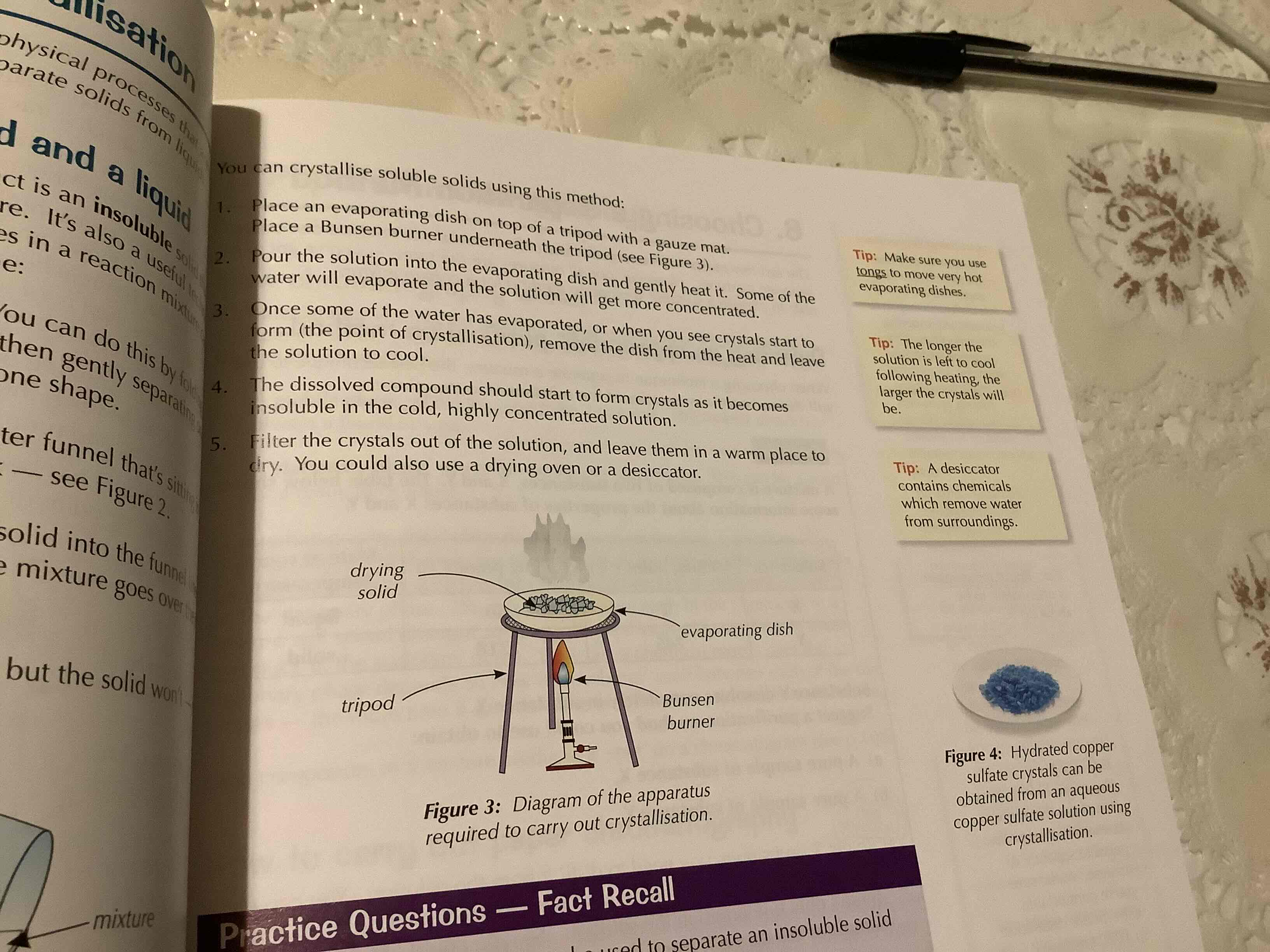
crystallisation
if a solid can be dissolved it is said to be soluble. you can use crystallisation to separate a soluble product from a solution
chromatography
chromatography is a method used to separate a mixture of soluble substances and identify them

Rf value
the ratio between the distance travelled by the dissolved substance (solute) and distance travelled by the solvent
how to calculate the Rf value
distance traveled by solute
distance travelled by solvent
process of water purification
filtration, sedimentation, chlorination

what is the ph scale
a measure of how acidic or alkaline a solution is, the pH scale goes from 0 to 14
pH of an acid
less than 7 (the lower the pH the more acidic)
pH of an alkali
greater than 7 (the higher the pH the more alkaline the substance is)
pH of a neutral substance
7, pure water is an example of a neutral substance
concentration of ions
The higher the concentration of hydrogen ions in a solution, the more acidic it is, so the lower the pH will be. So, as the concentration of hydrogen ions increase, the pH decreases. In alkaline solutions, the higher the concentration of OH- ions, the higher the pH