Organic Chemistry
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
Alcohol
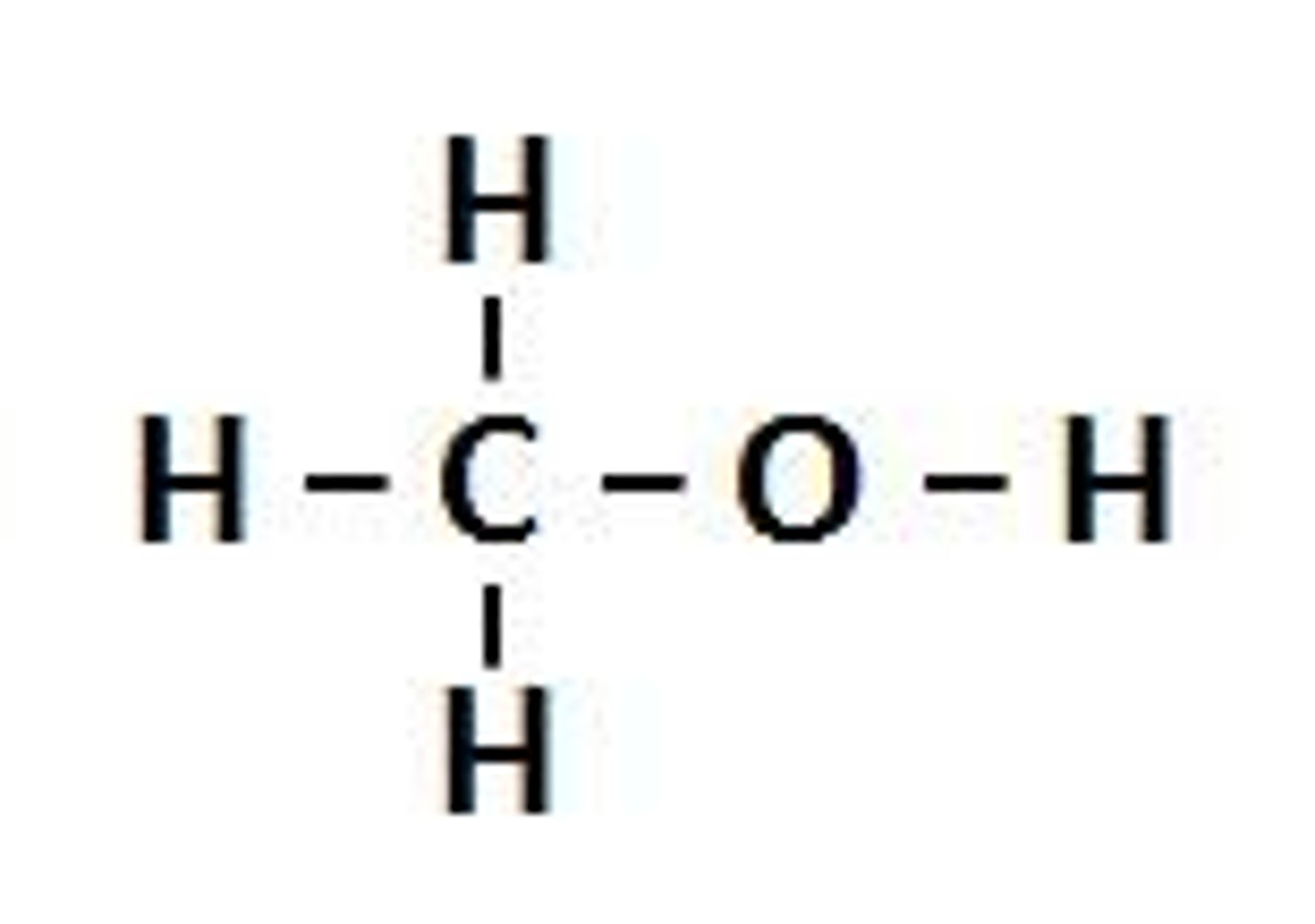
Ketone
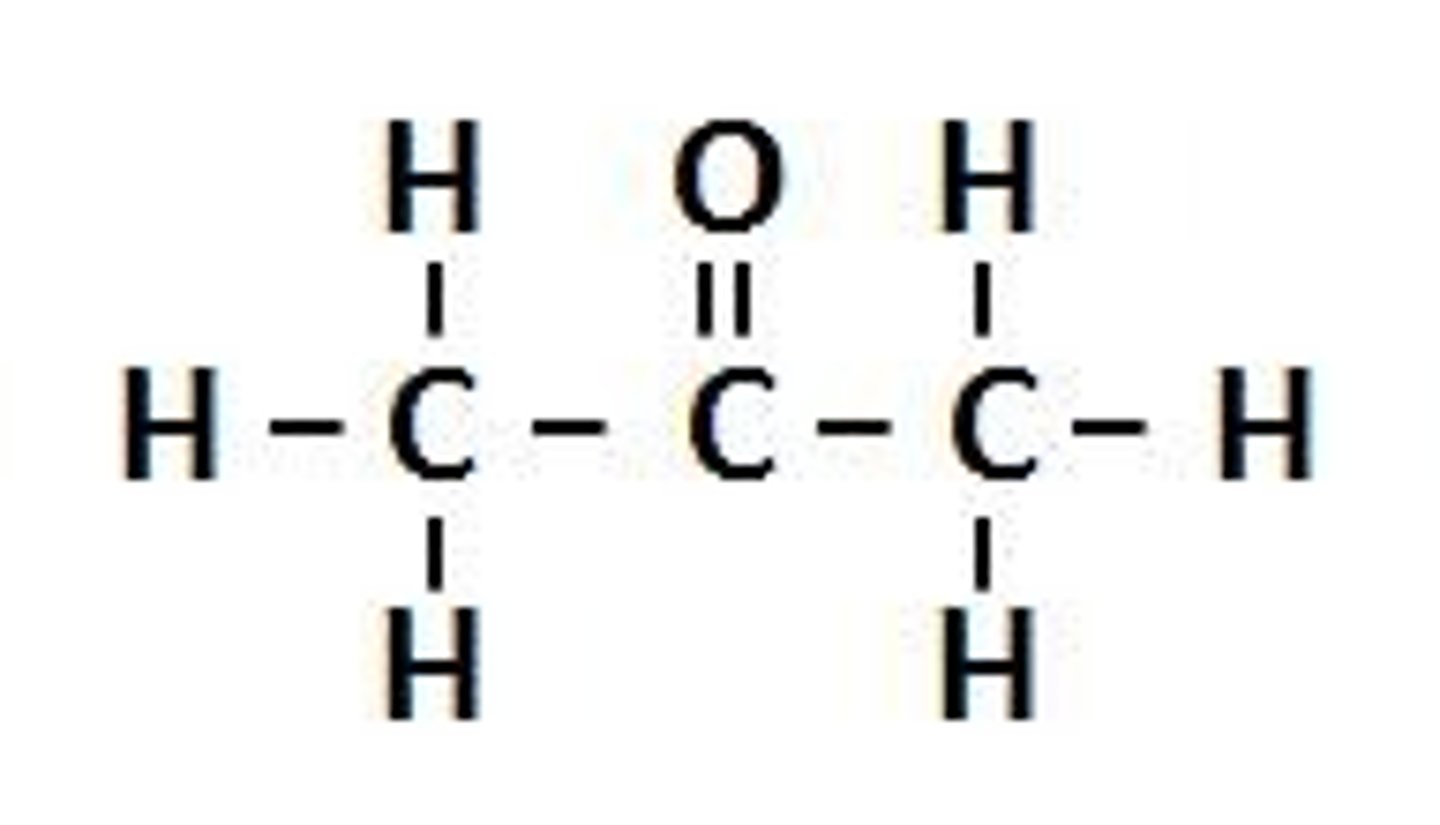
Carboxylic Acid
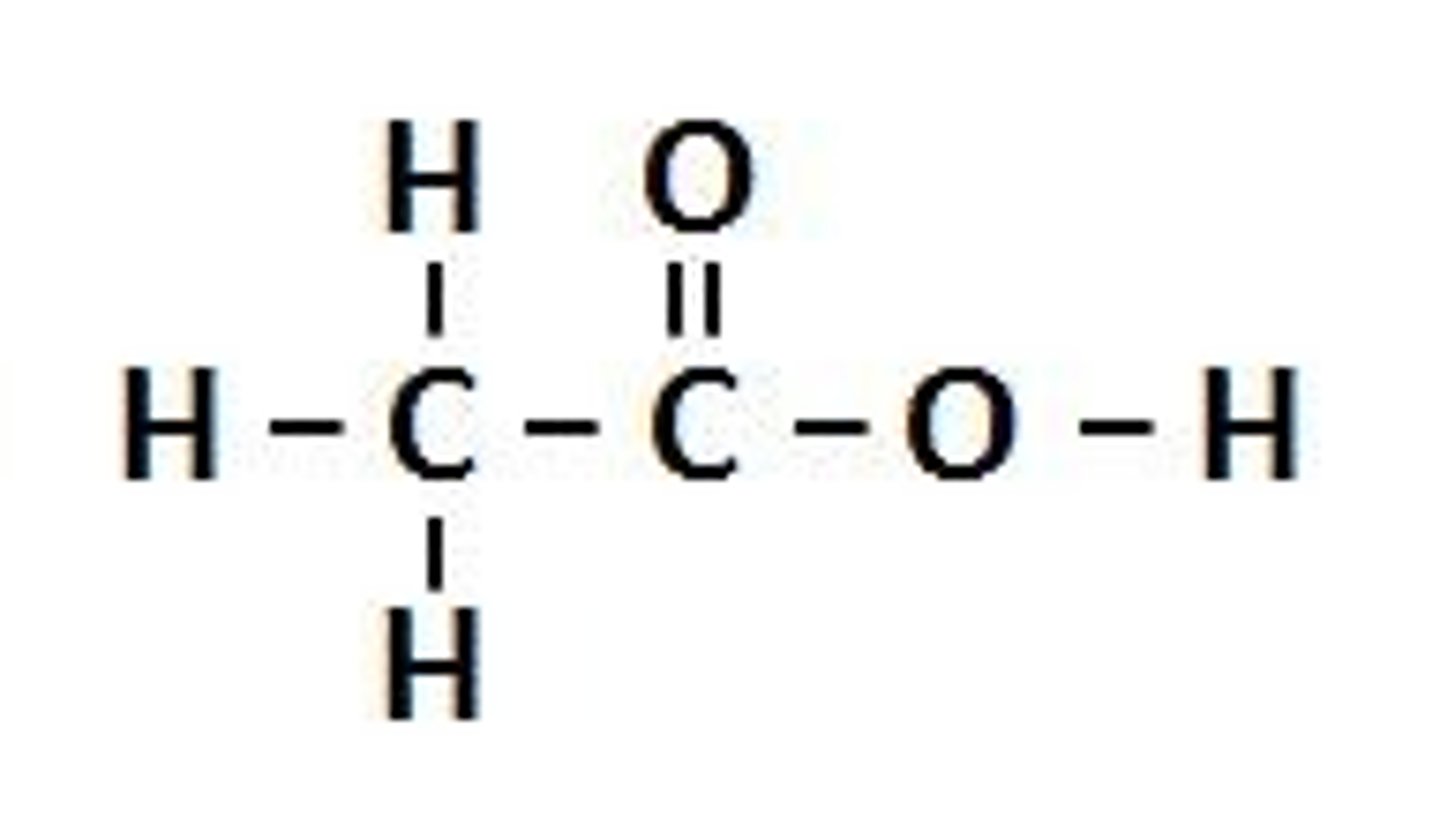
Ester
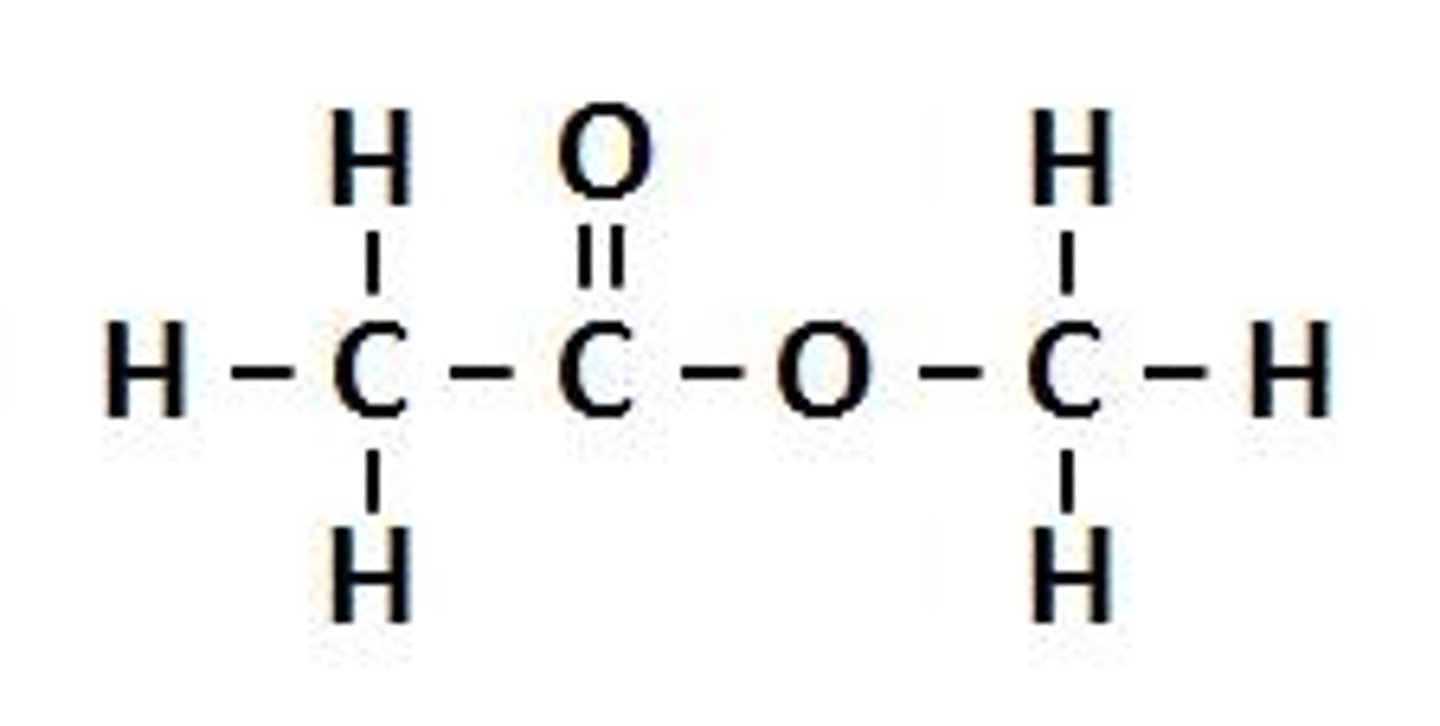
Aldehyde
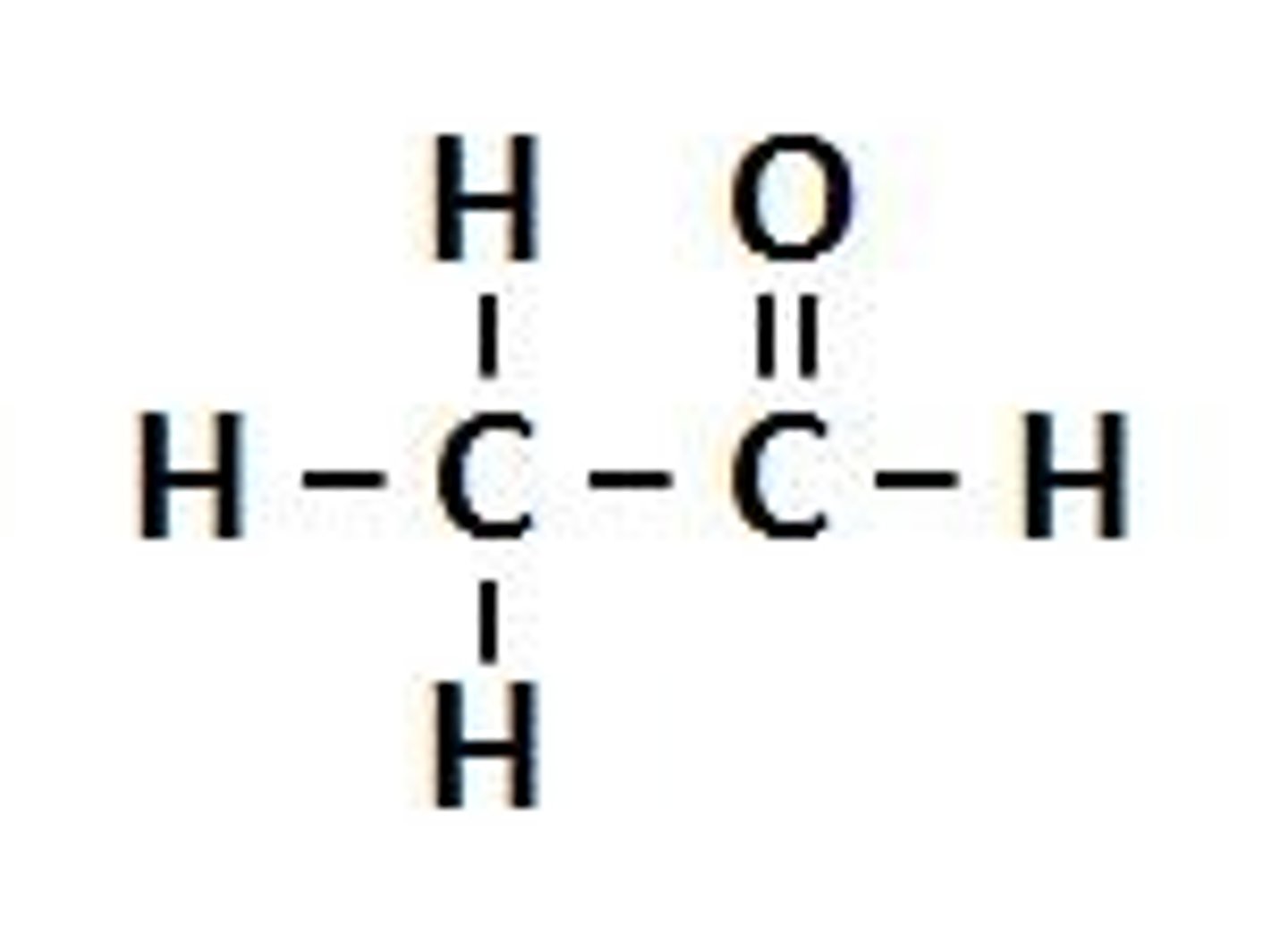
Amine
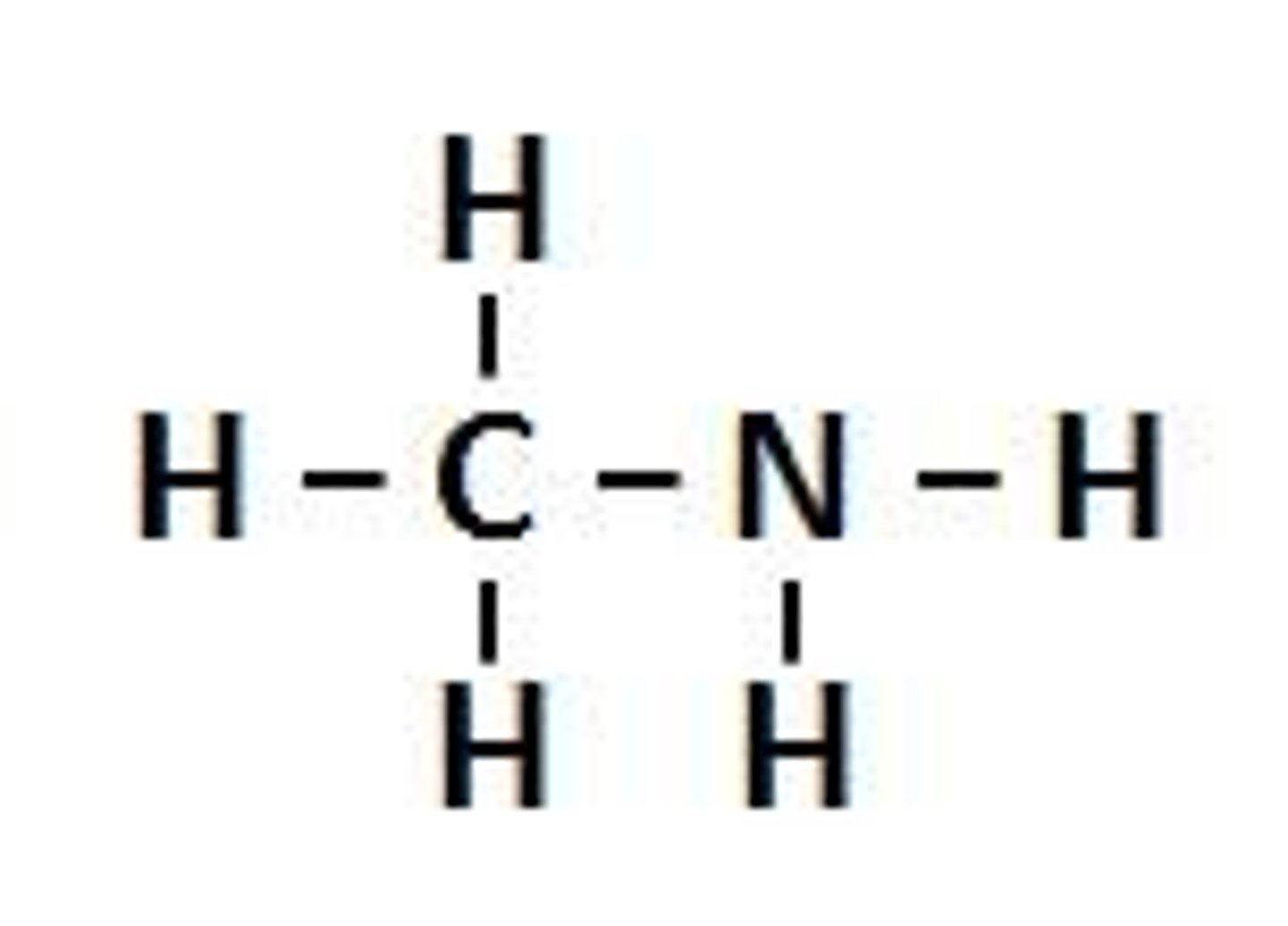
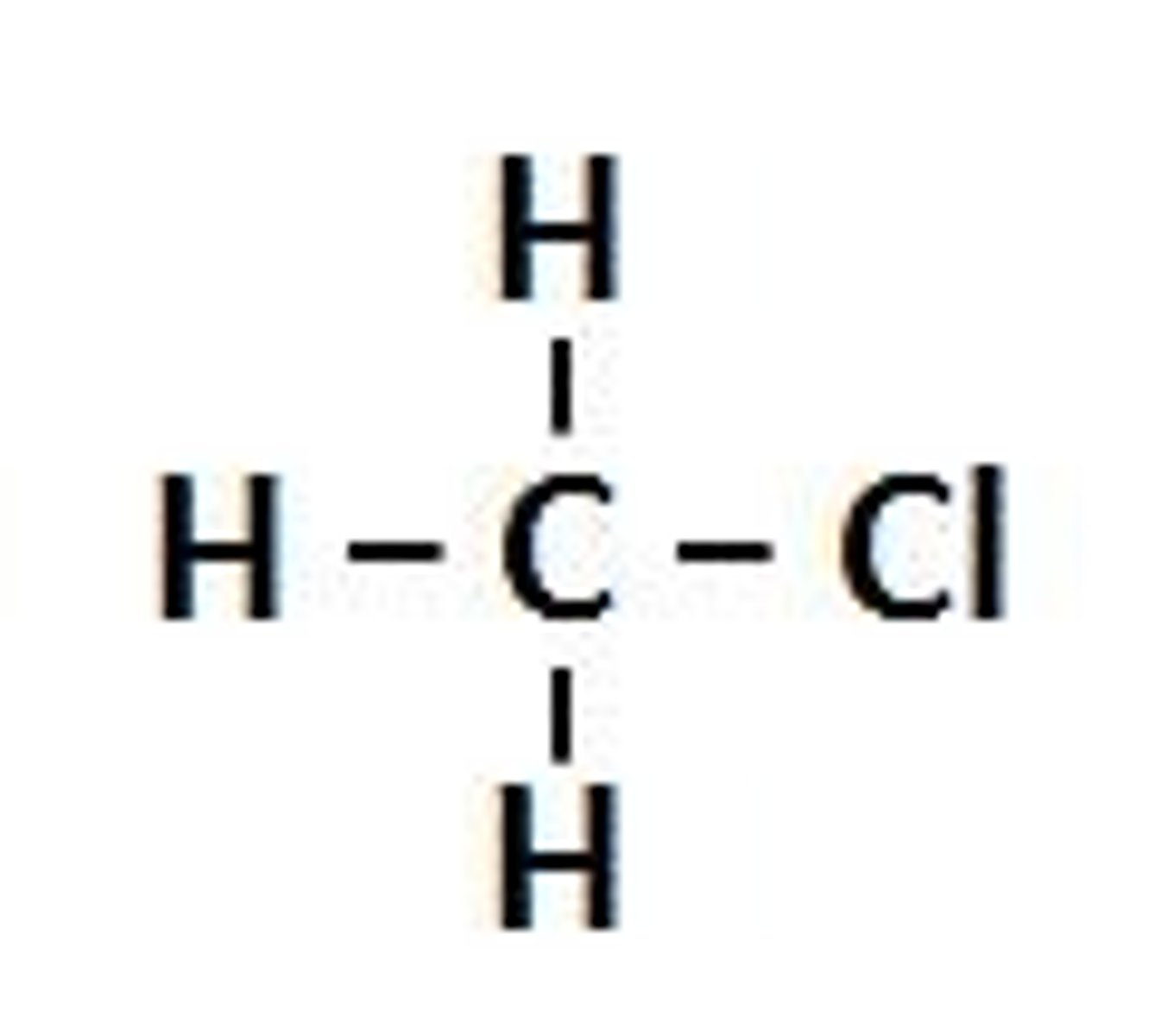
Alkyl halide
pentane
Name this.

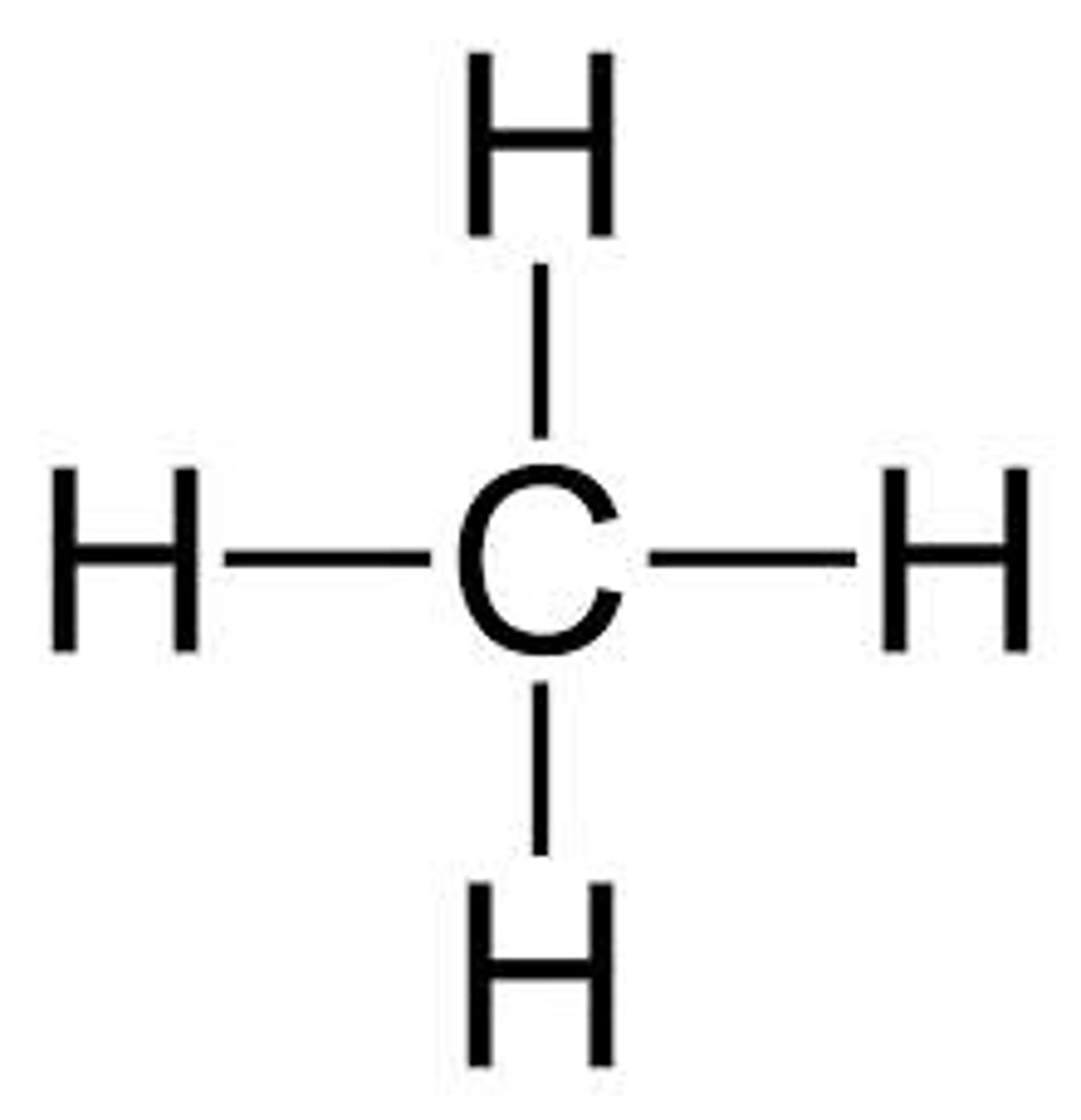
methane
Name this.
butane
Name this.

propane
Name this.
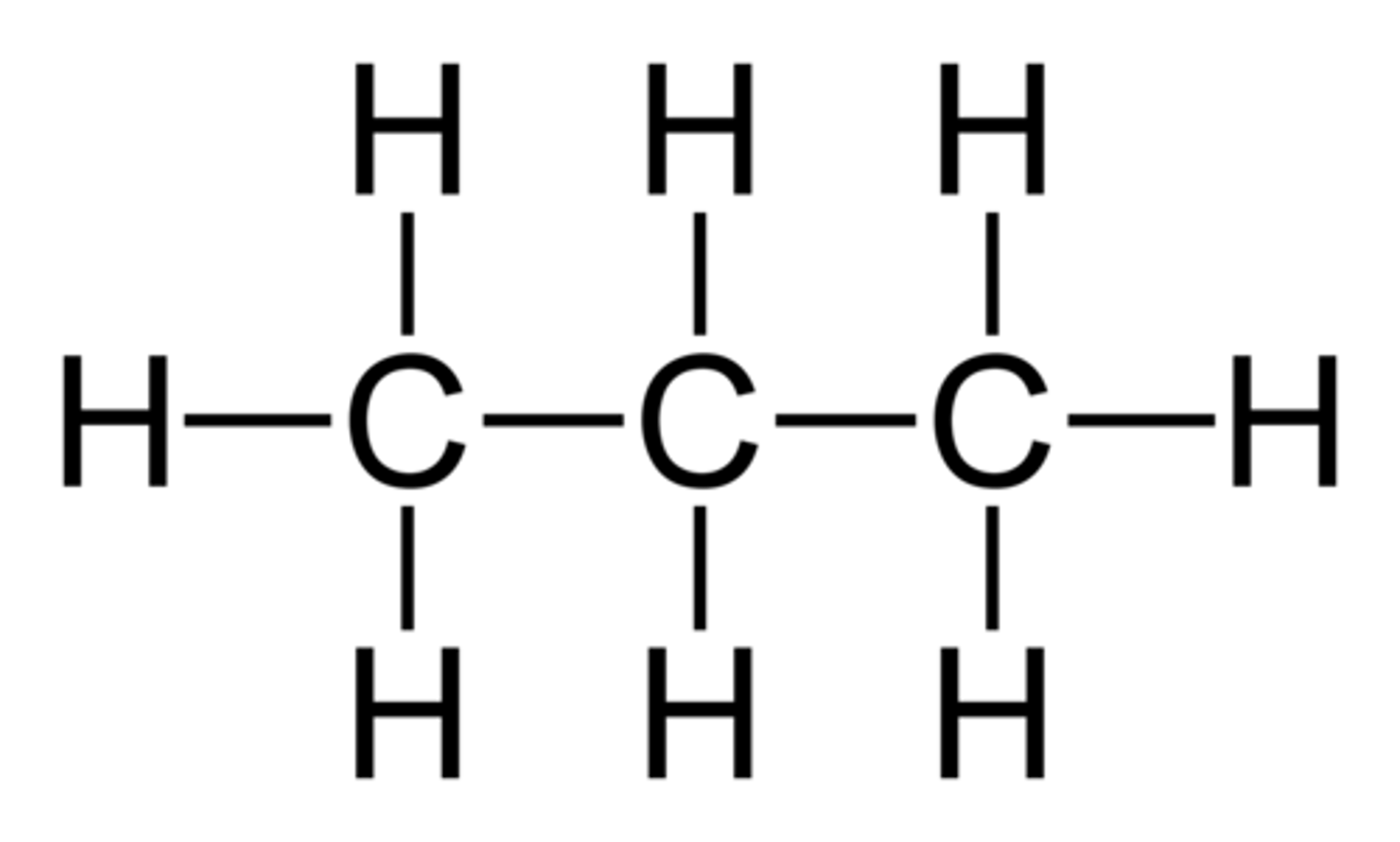
ethane
Name this.
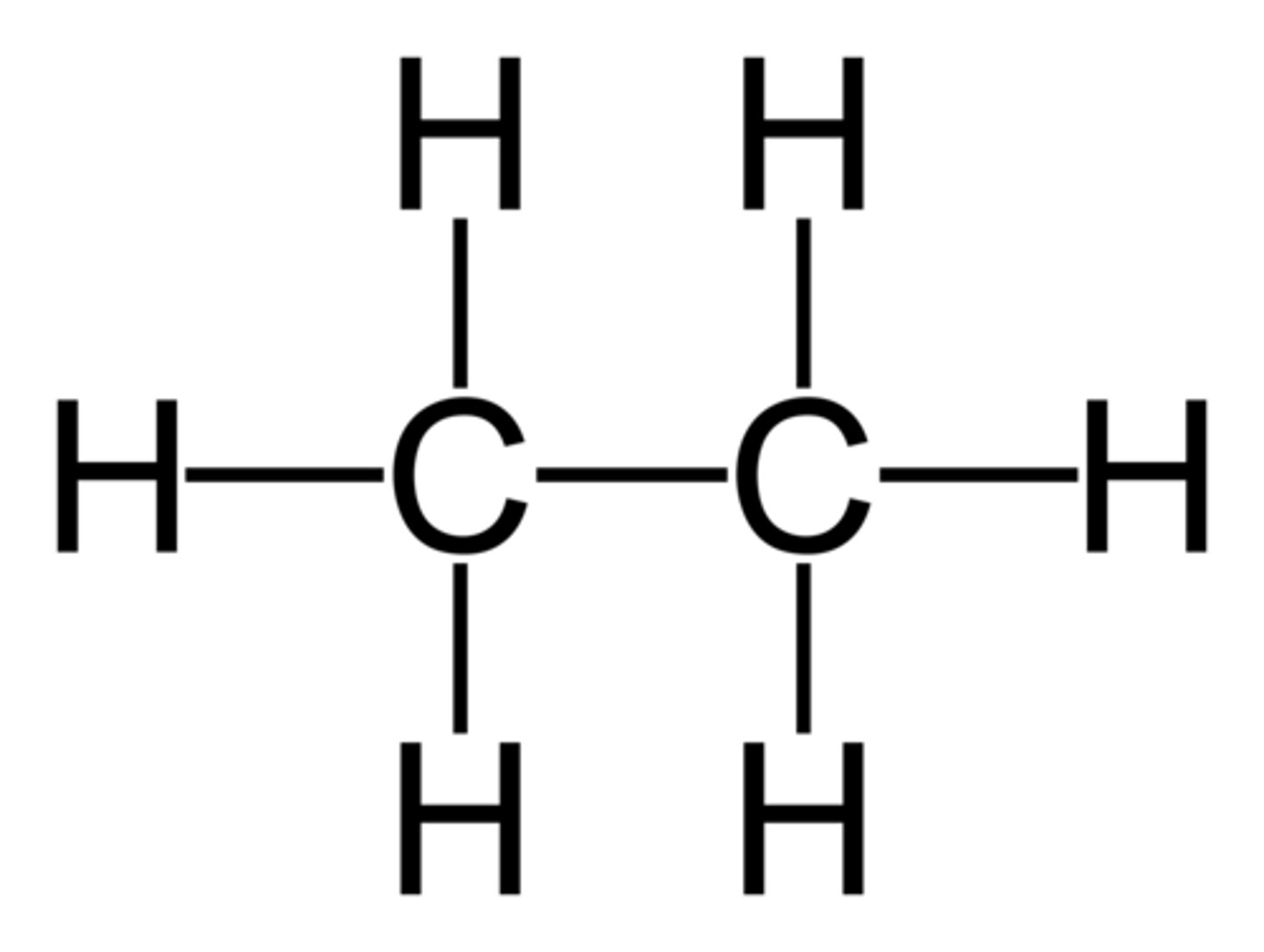
hexane
Name this.
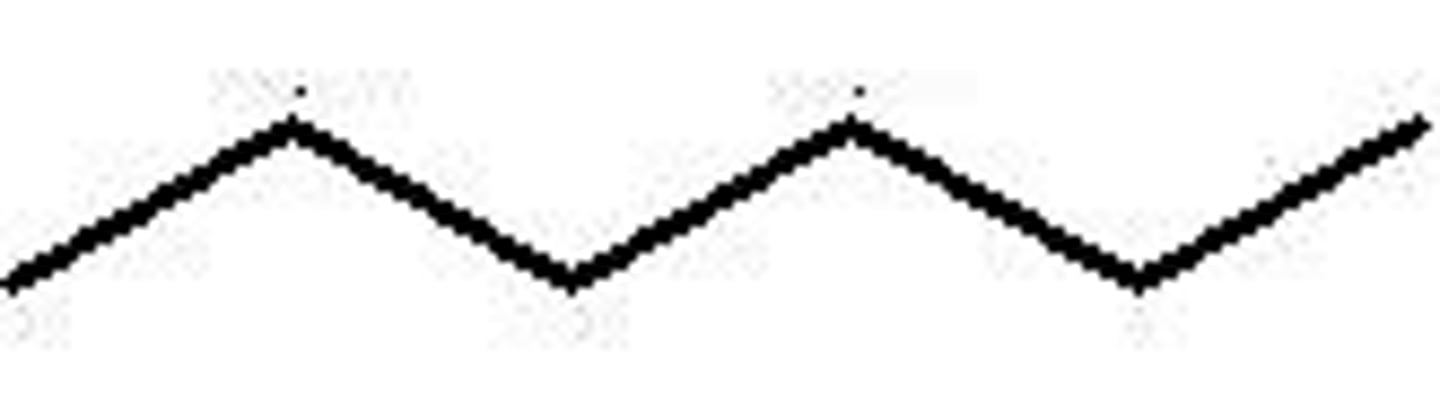
heptane
Name this.
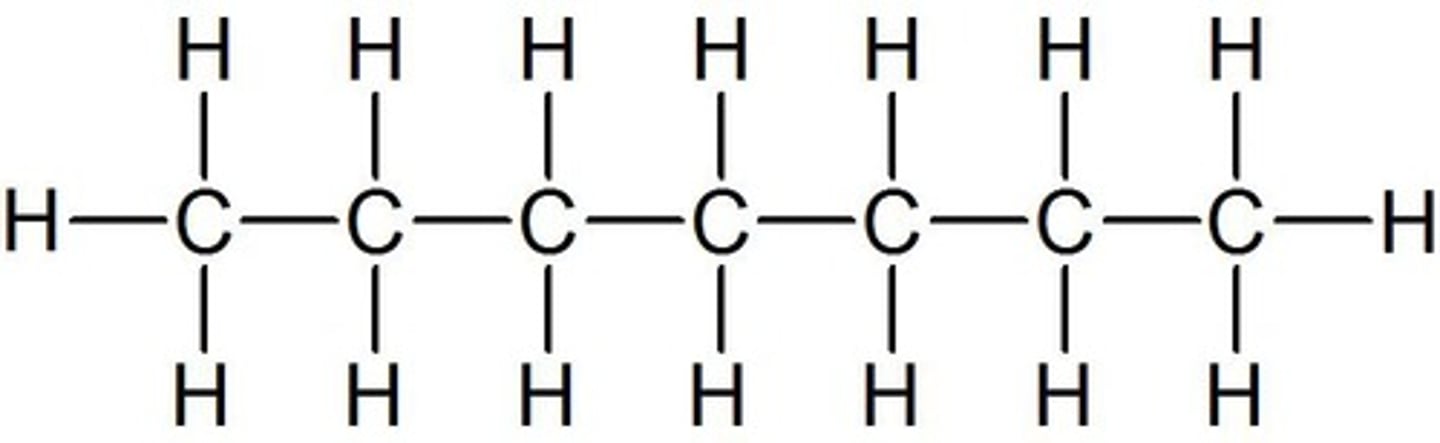
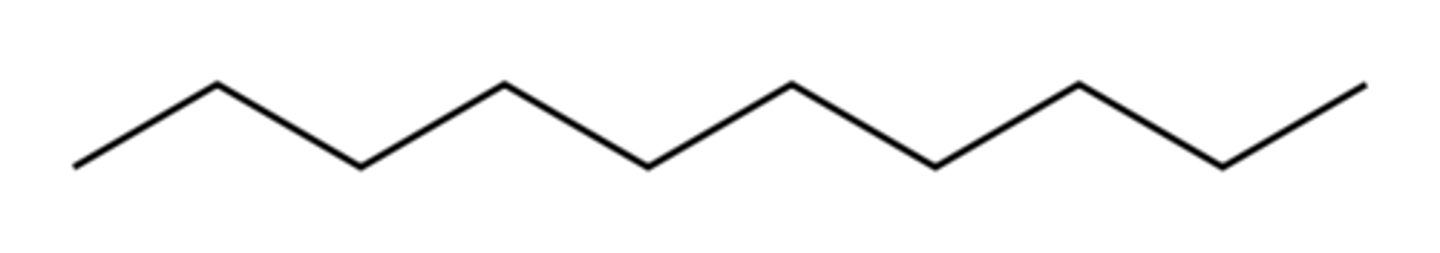
octane
Name this.
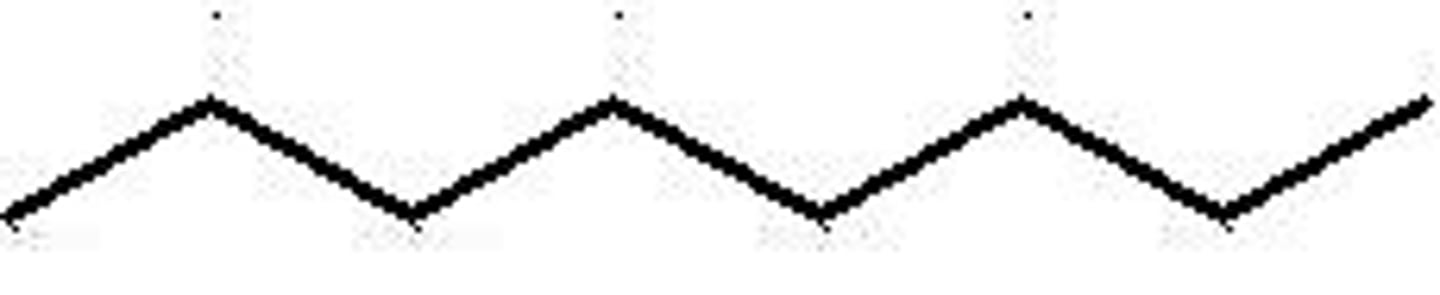
decane
Name this.
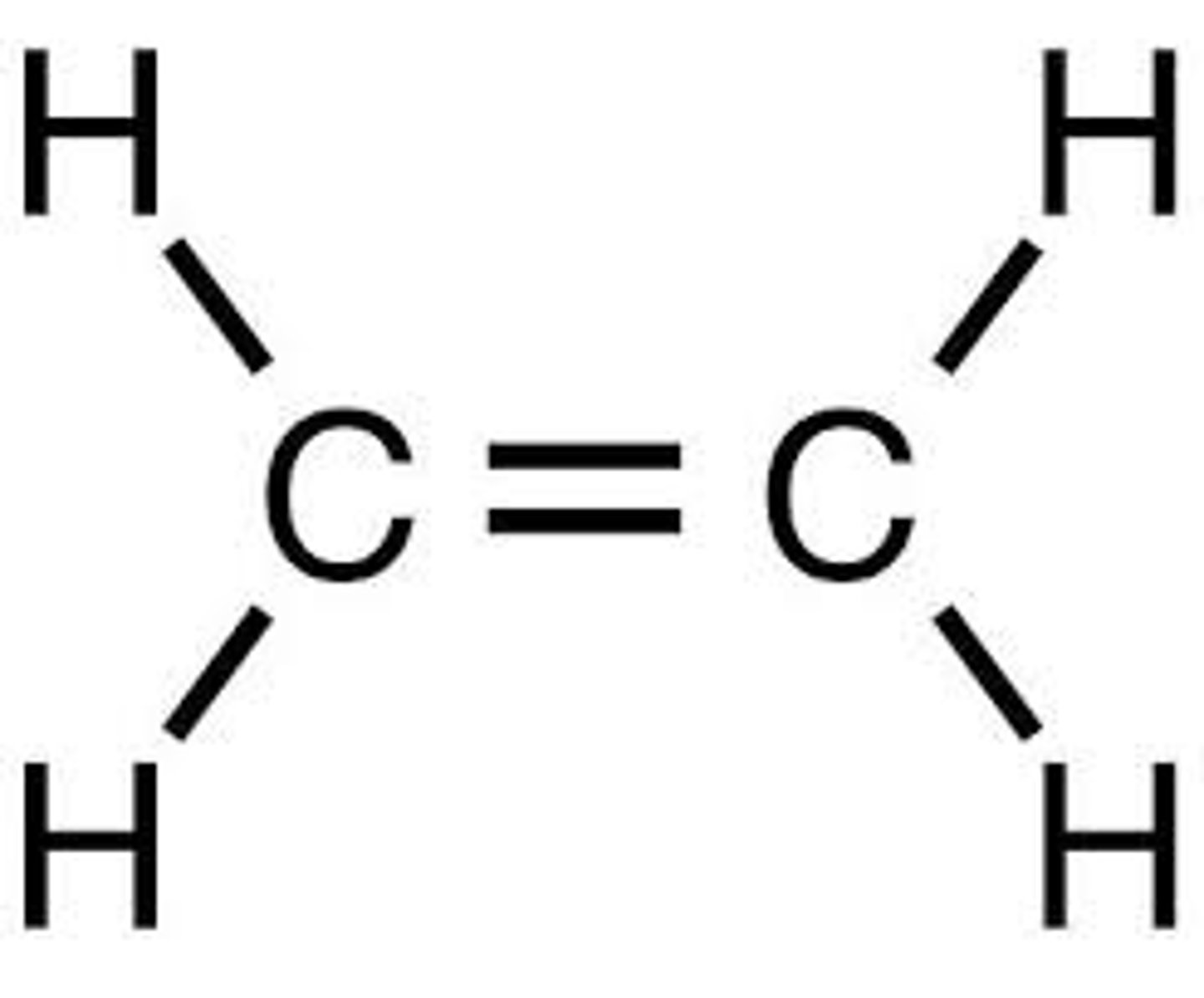
ethene
Name this.
propene
Name this.
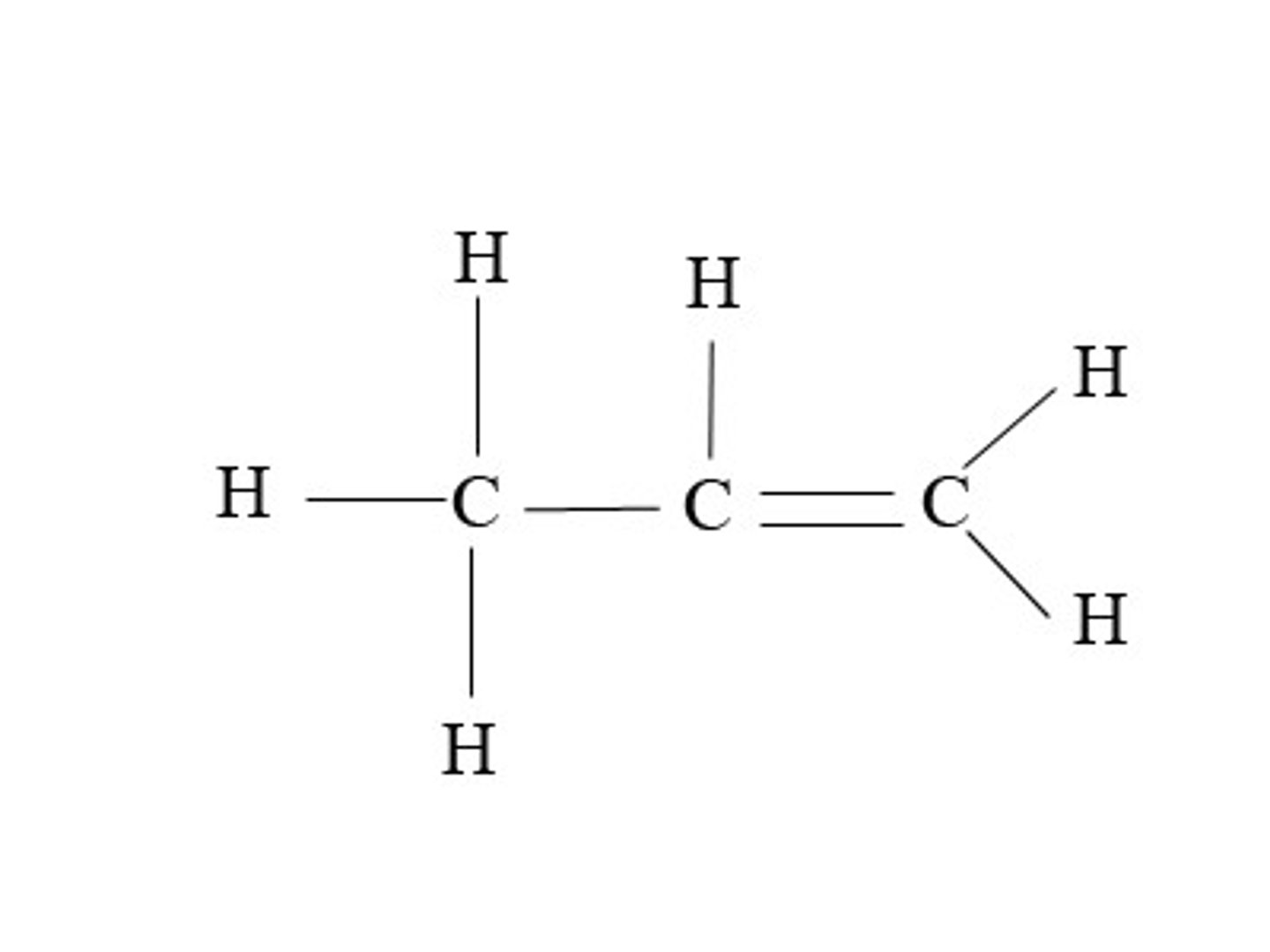
butene
Name this.
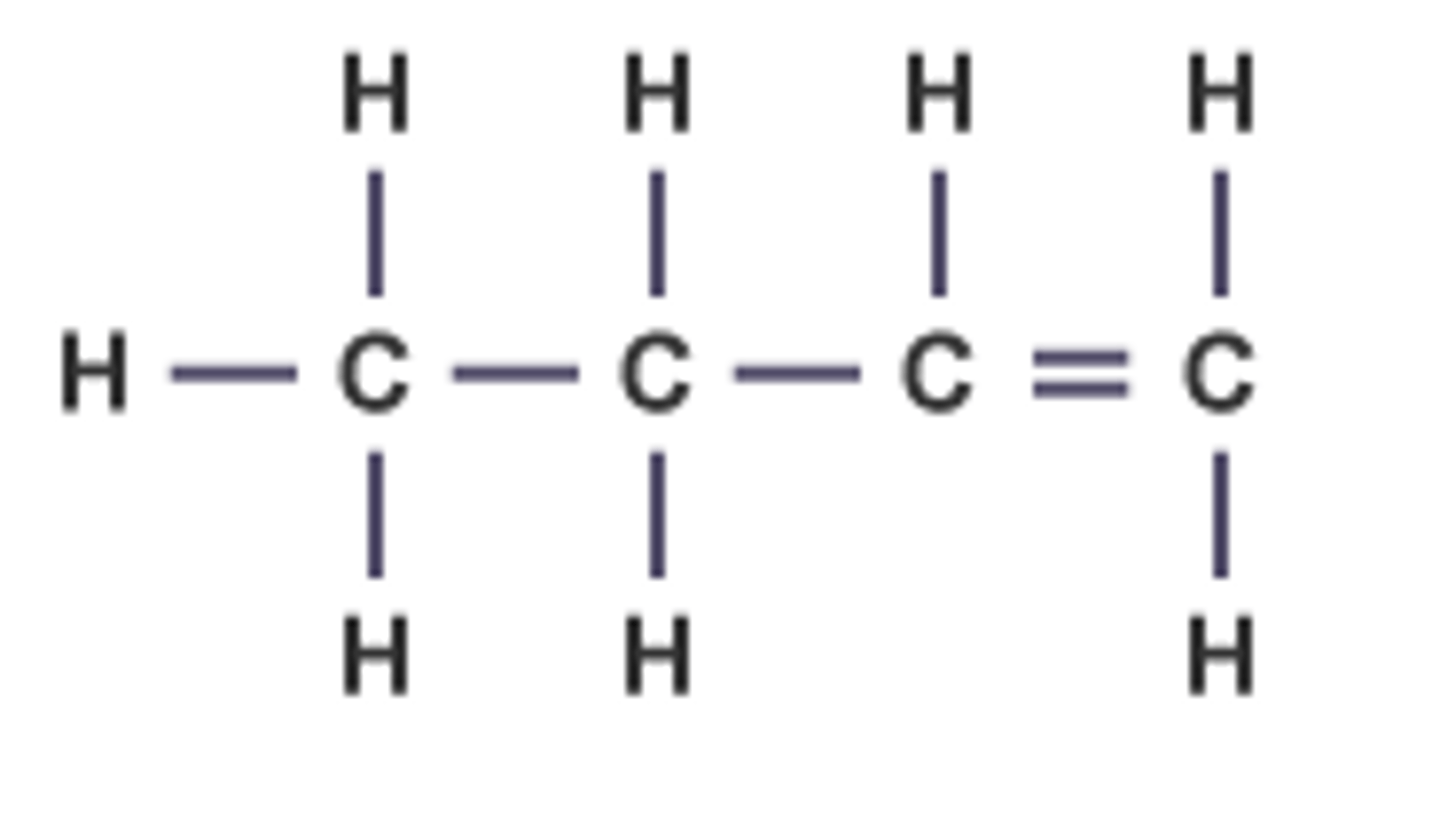
pentene
Name this.
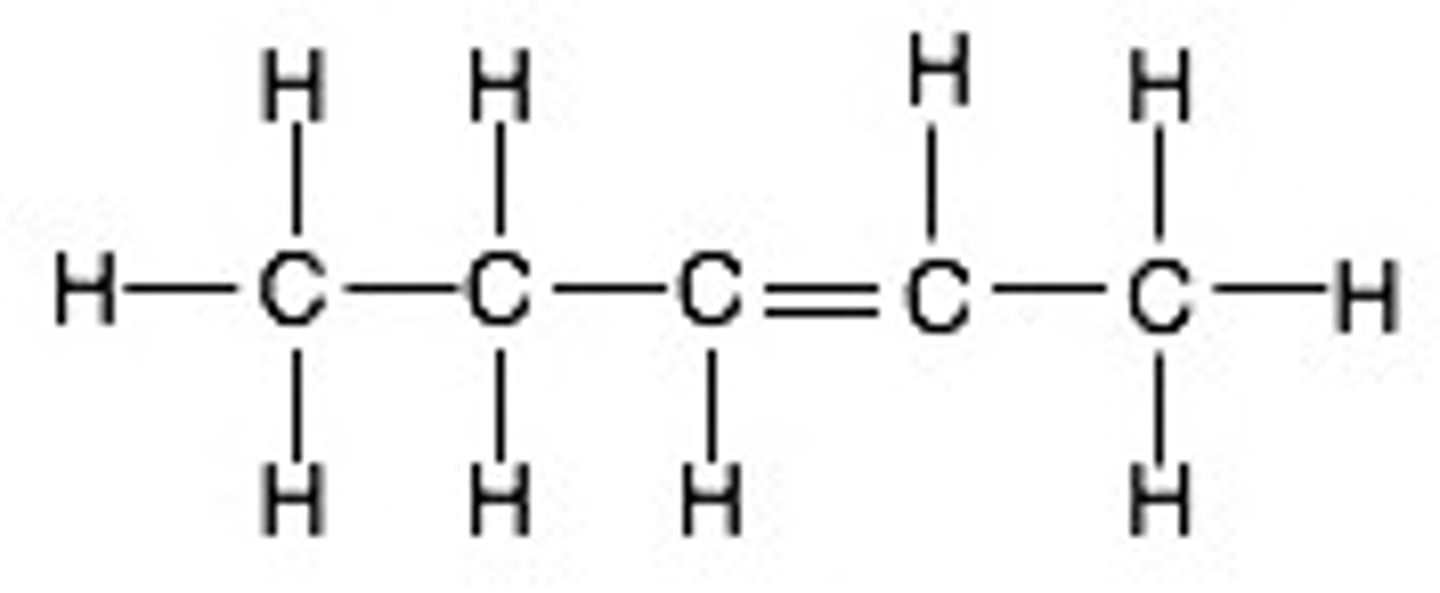
hexene
Name this.

4
The number of valence electrons Carbon has (number of bonds it could make.
triple bond
The strongest and shortest covalent bond
single bond
The weakest and longest covalent bond
CH4
molecular formula for methane
C2H6
molecular formula for ethane
C3H8
molecular formula for propane
C4H10
molecular formula for butane
C5H12
molecular formula for pentane
C6H14
Molecular formula for hexane
C2H4
Molecular formula for ethene
C3H6
Molecular formula for propene
C4H8
Molecular formula for butene
C5H10
Molecular formula for pentene
C6H12
Molecular formula for hexene
C8H18
Molecular formula for octane
C8H16
Molecular formula for octene
Saturated
A carbon chain that is filled up with the maximum capacity of hydrogens.
Unsaturated
A carbon chain that is not filled up with the maximum capacity of hydrogens but rather has double or triple bonds