Nucleophilic substitution at Saturated Carbon
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
How do the rate laws differ in Sn1 vs Sn2 nucleophilic substitution
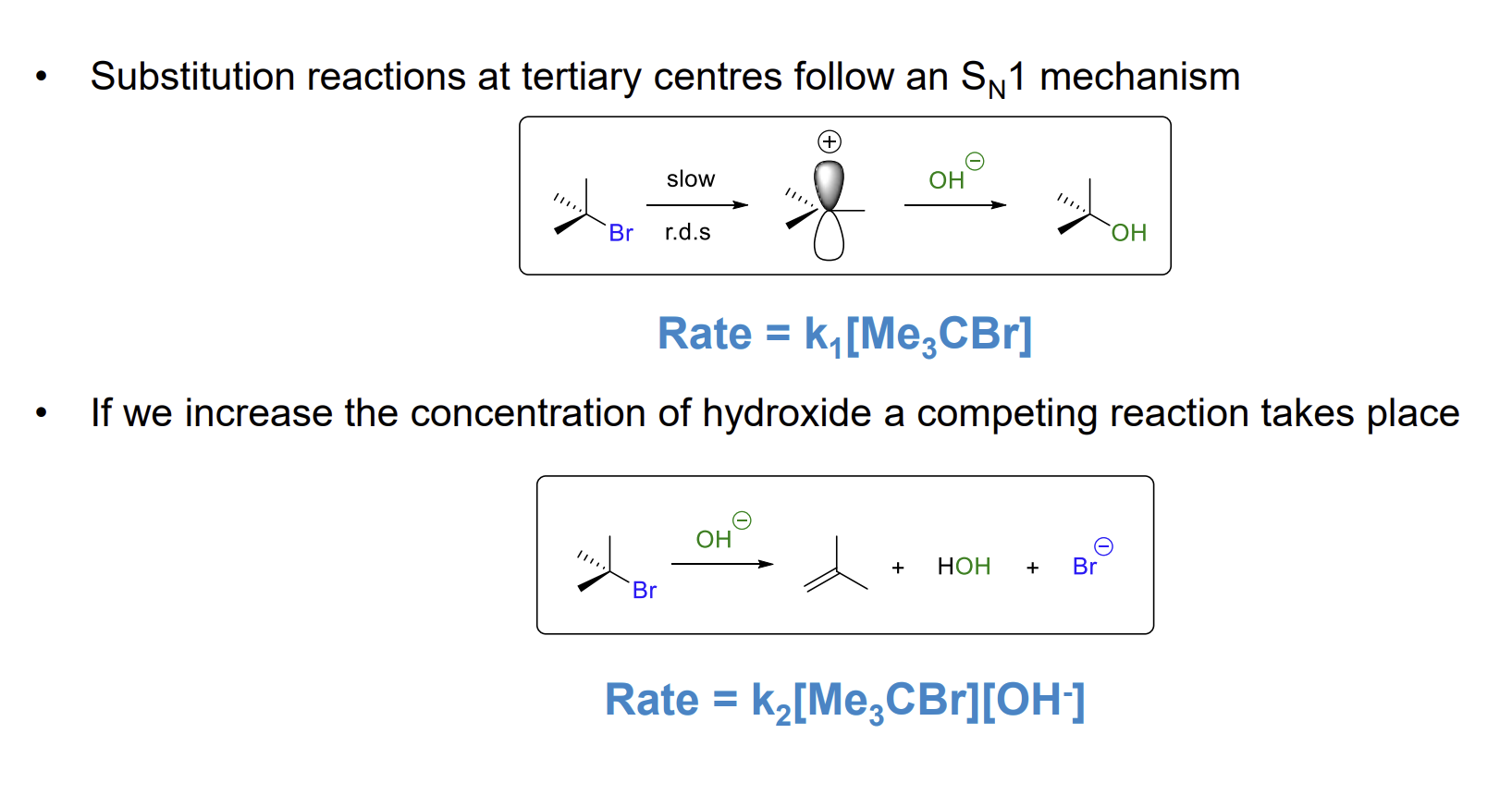
Sn1 is termed as such due to there only being one molecule in the rate equation

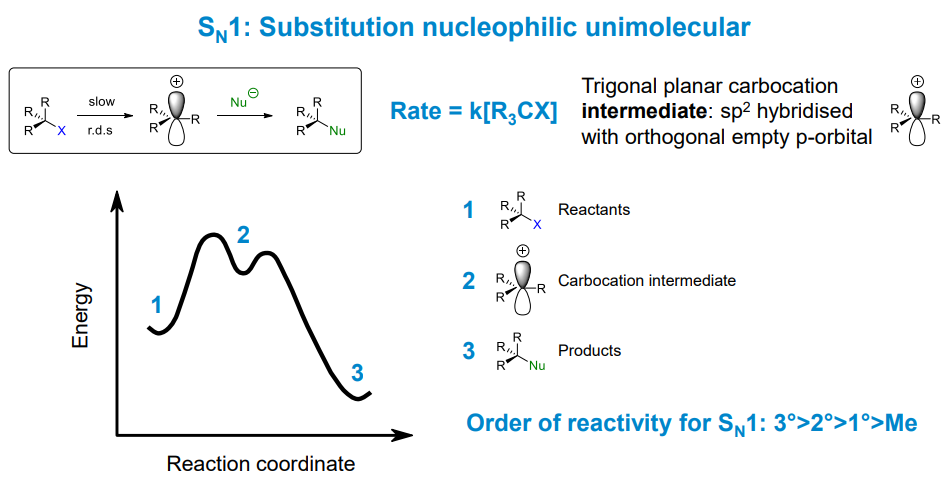
How does an Sn1 reaction proceed
It produces a carbocation intermediate (which can be observed)

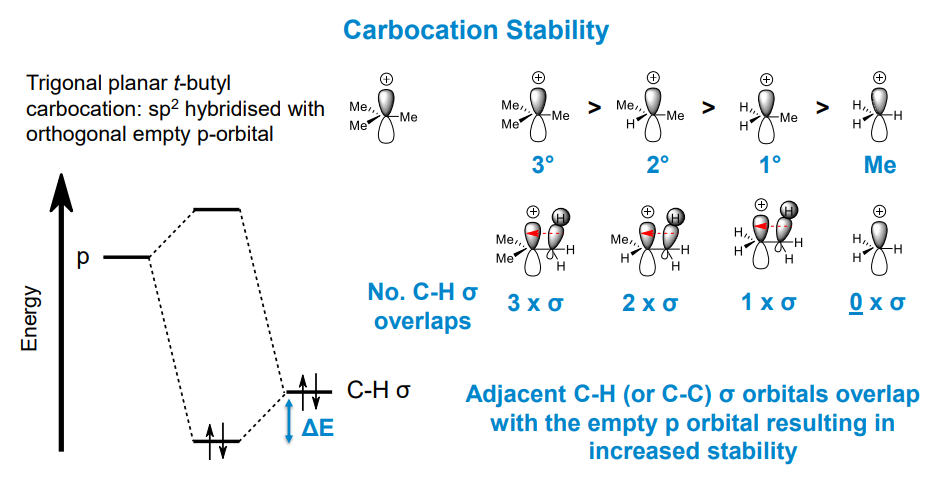
Why is this true?
Positive inductive effect of adjacent C-H sigma orbitals stabilises the positive change on the carbocation intermediate. Hence the more carbons the stronger the stabilising effect of overlapping sigma orbitals with the empty p-orbital.
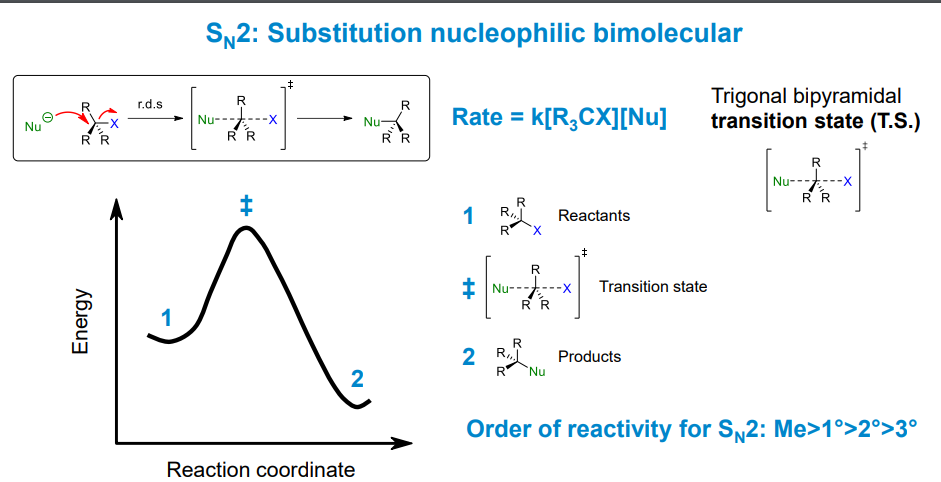
How does an Sn2 reaction proceed
There is no local minimum as there is only 1 step in the reaction involving both the nucleophile and electrophile. The transition state in this reaction is impossible to observe as it is too unstable
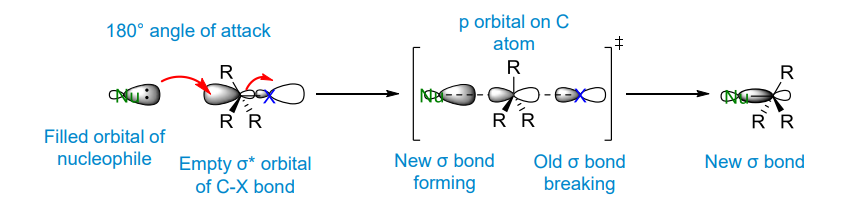
The nucleophile attacks the electrophilic centre from the back

Why is this true?
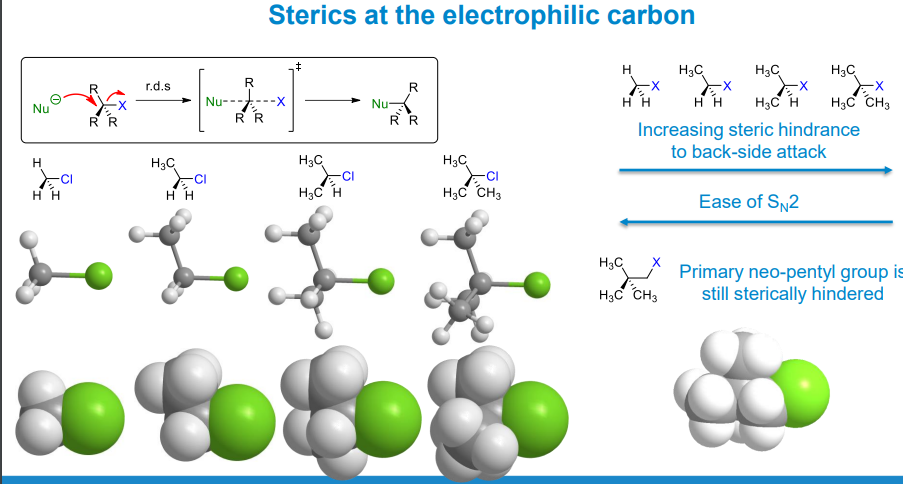
Steric hindrance is lower with smaller substituents on the carbon atom
What is Hammond’s Postulate
Essentially it means that the transition states will have similar structures to the carbocation intermediate.
Hence a more stable carbocation will lead to a more stable (still very unstable) transition state which reduces the activation energy of the reaction.
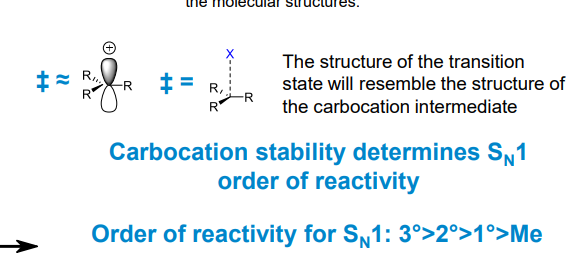
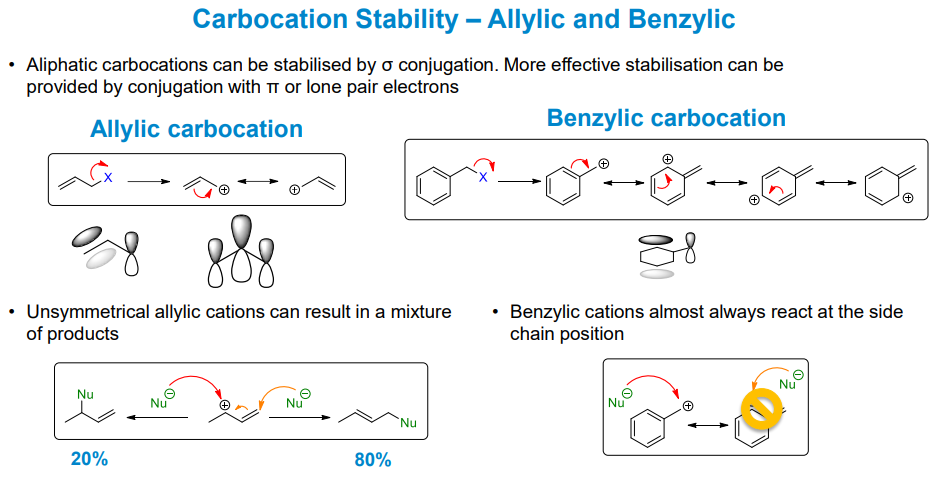
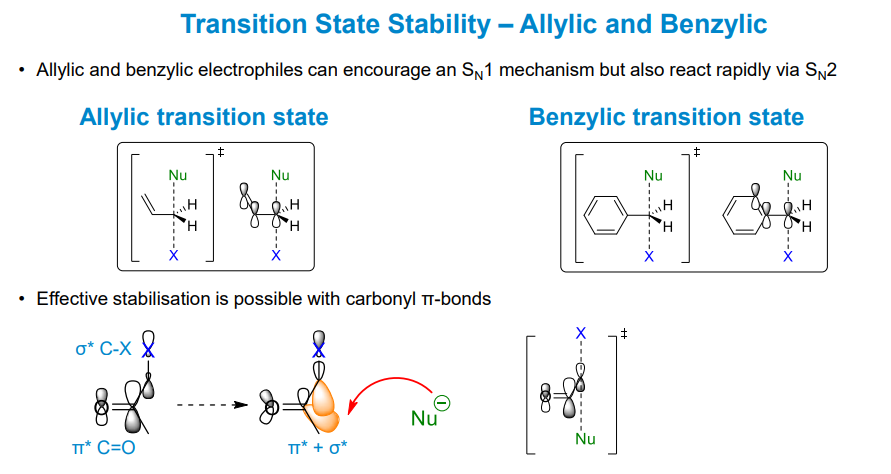
What other, more effective stabilising effects appear in carbocations
Allylic and benzylic conjugation from π orbitals, forming resonance forms which result in different products in some cases
How does the transition state form in an Sn2 reaction
HOMO to LUMO donation into an antibonding orbital. The transition state is the point at which the new bond is forming and the old bond is breaking
What determines Sn2 reaction rate
Steric hindrance - essentially the physical size of the substituents surrounding the electrophilic centre.
Do allylic groups favour Sn1 or Sn2
Neither by any large margin
While the conjugation stabilises carbocation intermediates, the pi orbitals can stabilise the p orbital on the carbon and stabilise the transition state
Benzylic and carbonyl groups can also stabilise the transition state in Sn2 reactions. The carbonyl group is the best at this, combining antibonding orbitals to make the carbon atoms involved more electrophilic.
What are the stereochemical implications of Sn1 reactions
Racemic mixture is formed as there is equal odds of attack above or below the plane of the carbocation intermediate.
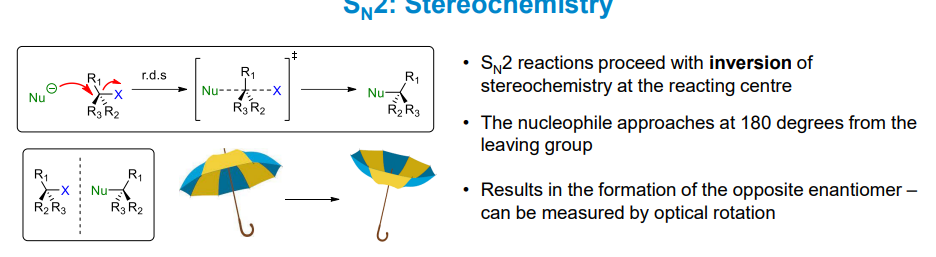
What are the stereochemical implications of Sn2 reactions
The stereochemistry is inverted at the reaction centre. Since the nucleophile always attacks at 180 degrees to the leaving group, the opposite enantiomer is formed as the substituent is now on the opposite side of the carbon.
You can liken the process to applying a strong force to the underside of an umbrella which will invert it.
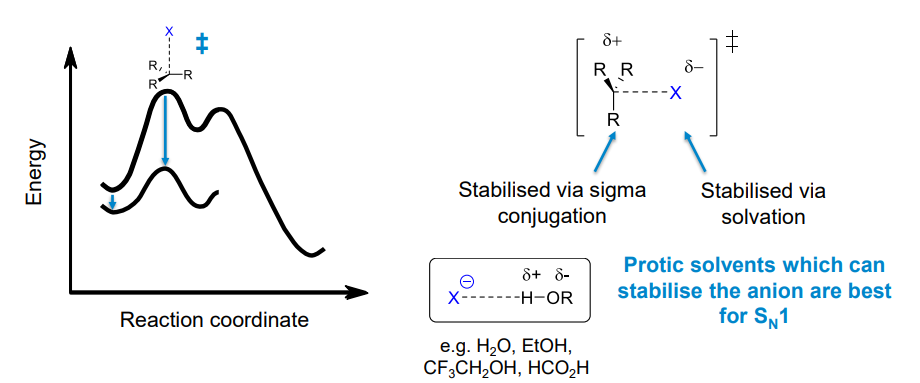
Which solvents favour Sn1 reactions
Polar, protic solvents as it stabilises the first transition state by stabilising the leaving group
What is the dielectric constant ε
A measurement of solvent polarity
It is a measure of the ability of a solvent to screen the electrostatic attraction between opposite charges (essentially ability to separate the charges from each other, such as separating a carbocation form a leaving group anion)
Dielectric constant effect on Sn1 reaction rate
Generally a higher dielectric constant means a faster rate of Sn1 reaction. However, a very important factor to consider is whether the solvent is protic or not. Protic solvents are much better at stabilising the transition state so may increase the rate of reaction more than a polar aprotic solvent despite having a lower dielectric constant.
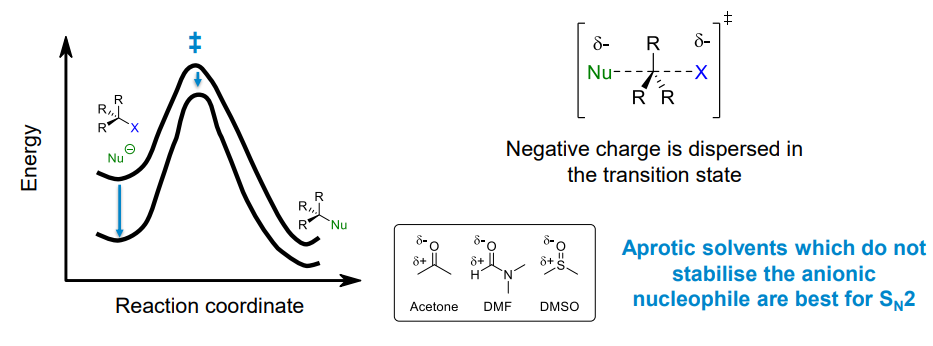
Effect of solvent polarity on Sn2 reaction rate
For Sn2 reactions with a negatively charged nucleophile, more polar solvents will have a slower reaction rate.
This is because the solvent will stabilise the Nu- more than the transition state as the charge in the transition state is more dispersed. Hence the activation energy increases
Aprotic polar solvents are better for Sn2 reactions (ig because non-polar solvents cause solubility issues)
What is a naked anion
Anions in aprotic polar solvents are only weakly solvated (stabilised).
Aprotic solvents also effectively solvate the counter-cation of the anion
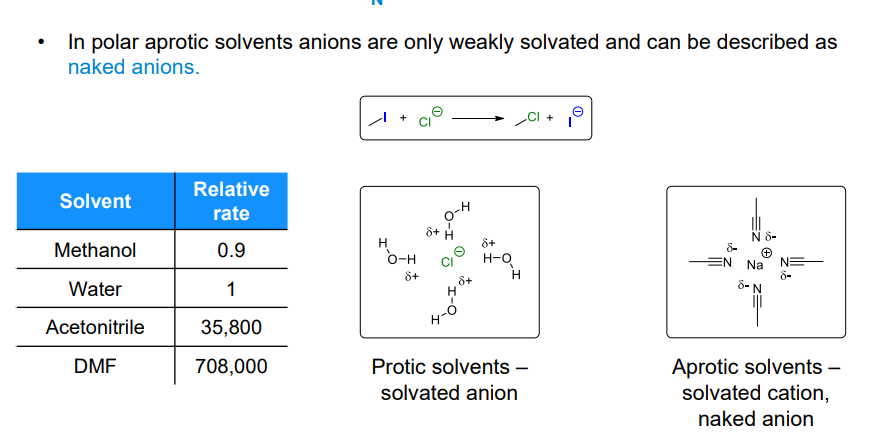
What affects the leaving group ability
Strength of the C-X bond
Stability of X-
The stability of X- can be predicted using pKa. A lower pKa implies that the conjugate base i.e. X- is more stable.
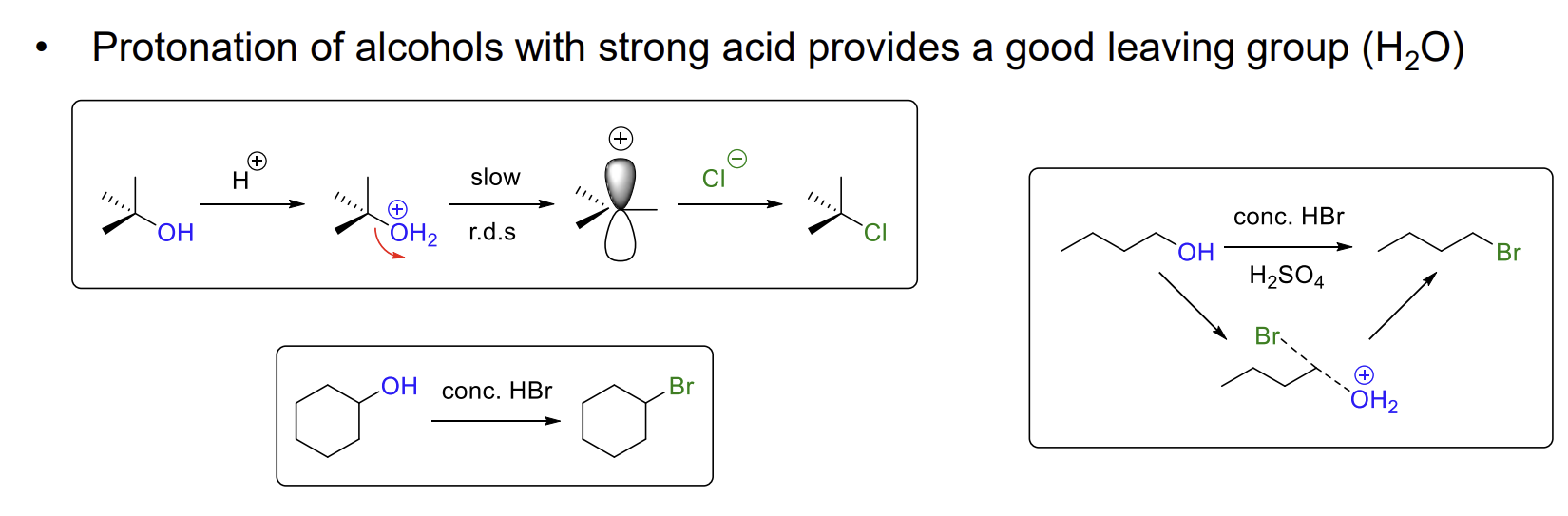
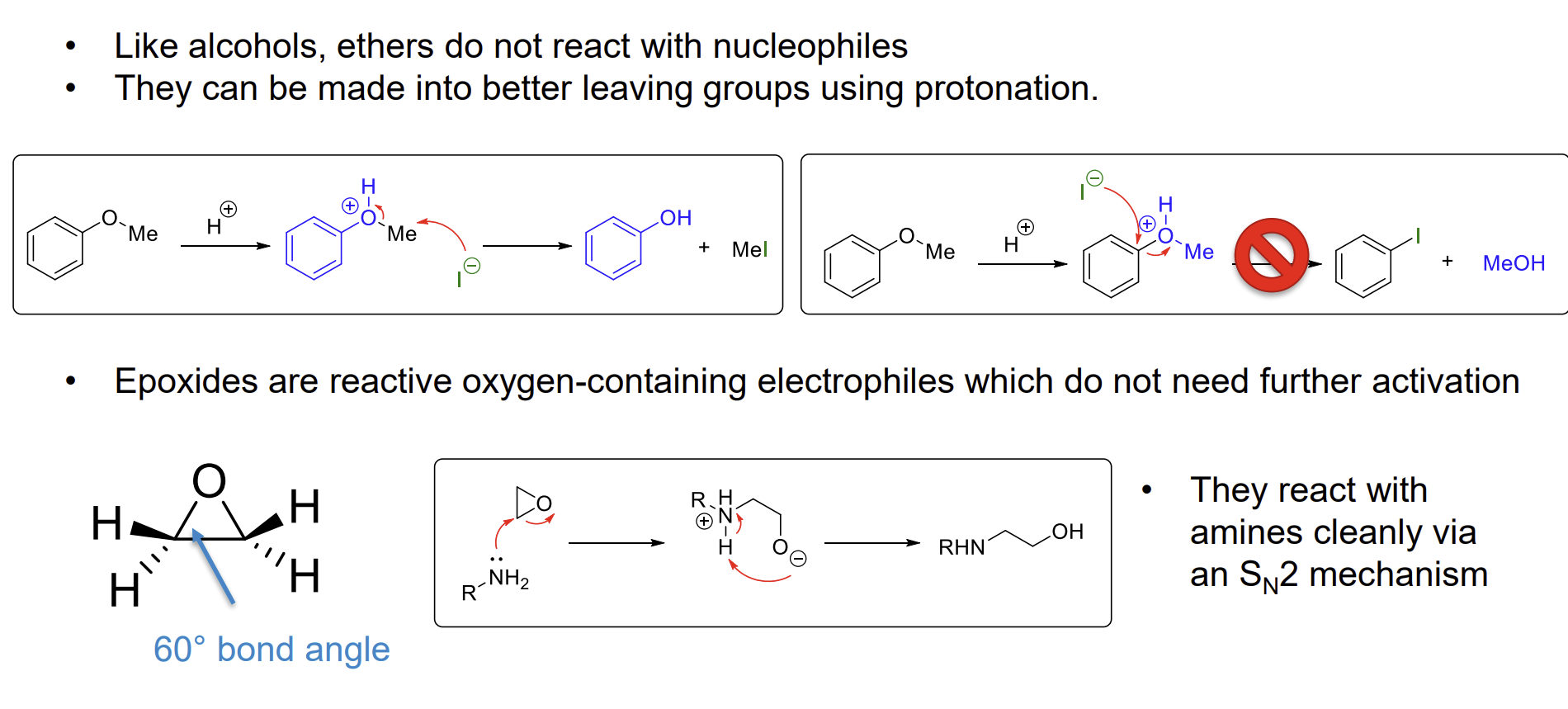
Why do alcohols not react with nucleophiles
OH- is a poor leaving group as it is basic (high pKa)
How can we make alcohols a better leaving group
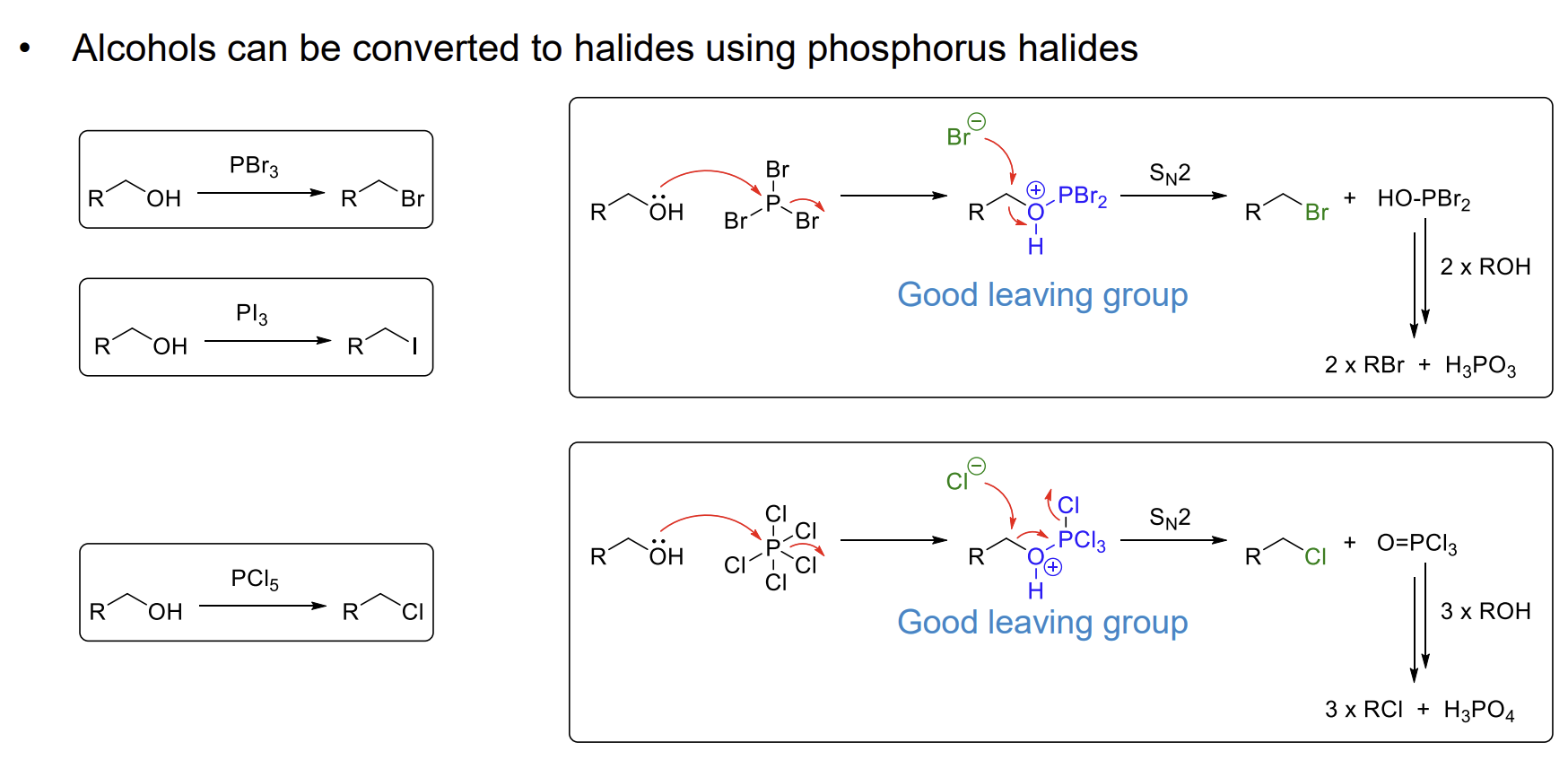
How do we convert alcohols to halides
Using phosphorus halides.
PCl5 is used as PCl3 is corrosive
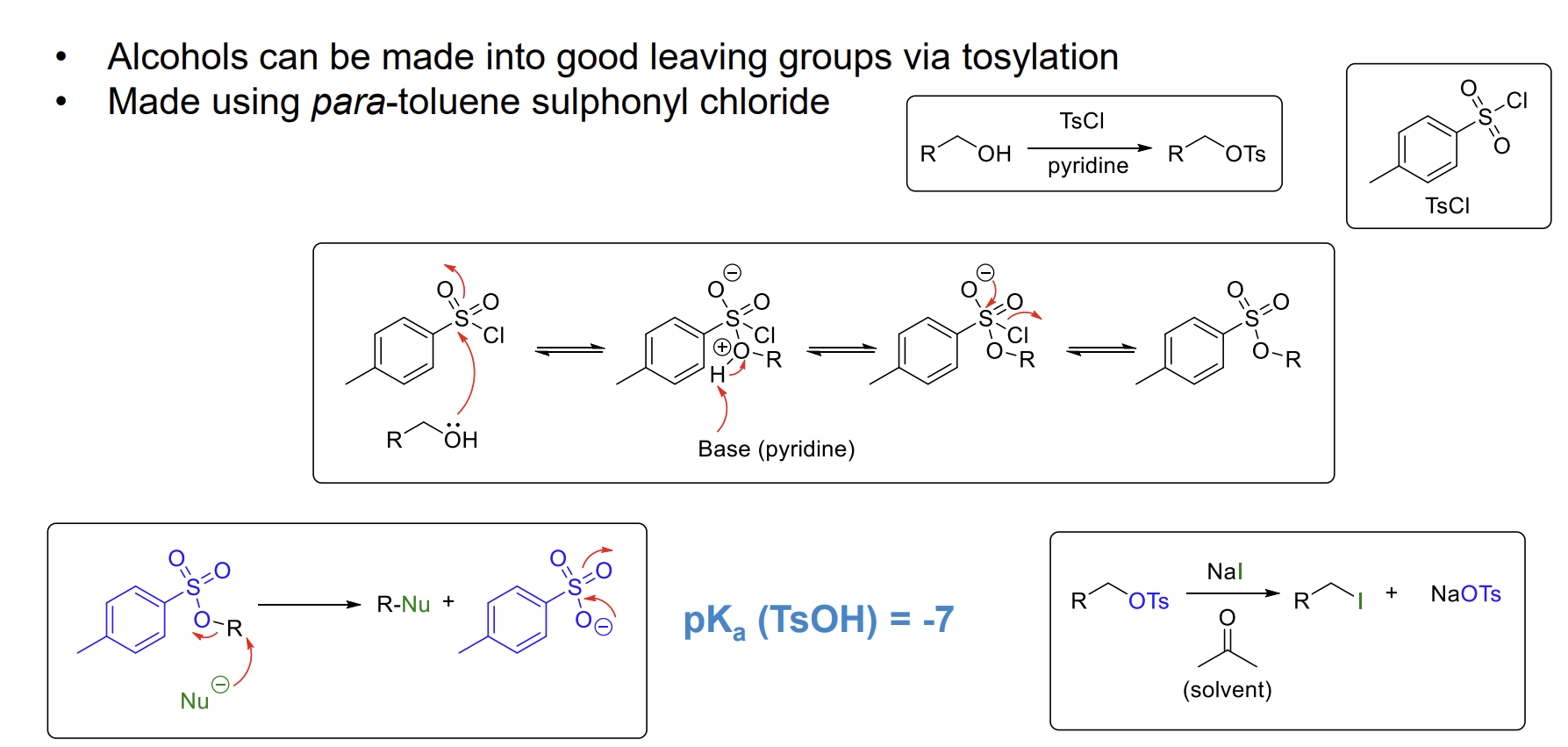
What is another way to make alcohols a better leaving group
Tosylation
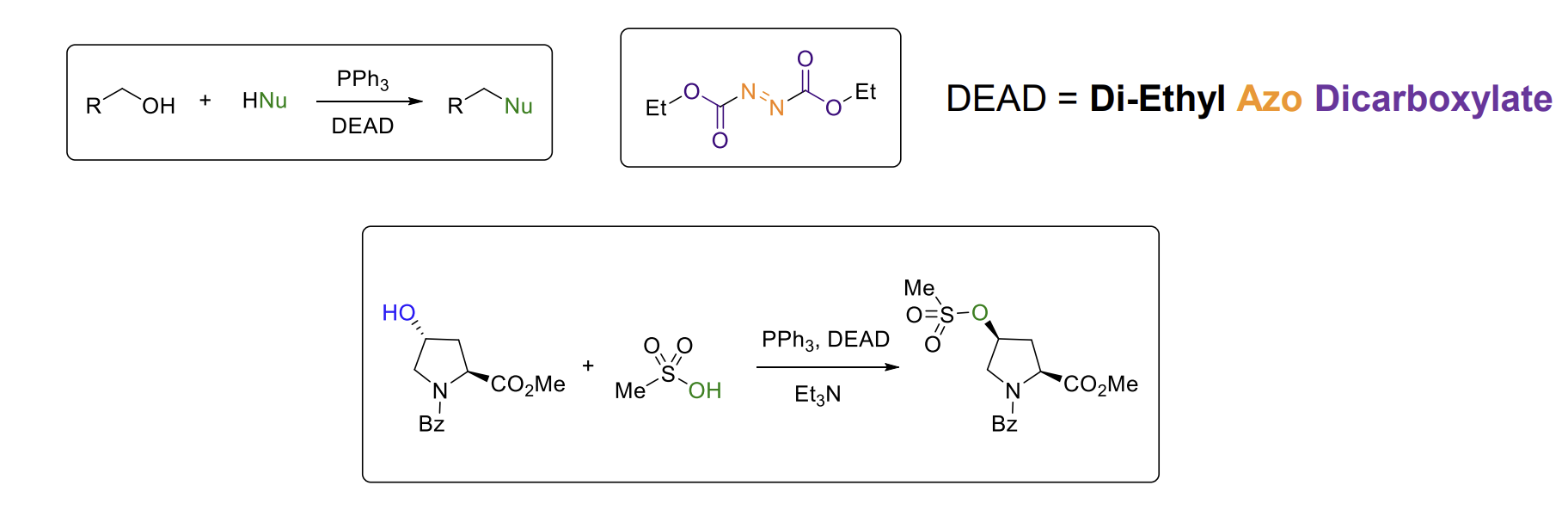
Mitsunobu reaction
Allows you to put an alcohol into a reaction mixture and get an SN2 product
How can we use ethers as electrophiles
How do nucleophiles affect Sn1 reactions
Strength of the nucleophile compared to the solvent can affect the product
How do nucleophiles affect Sn2 reactions
It determines the rate of reaction
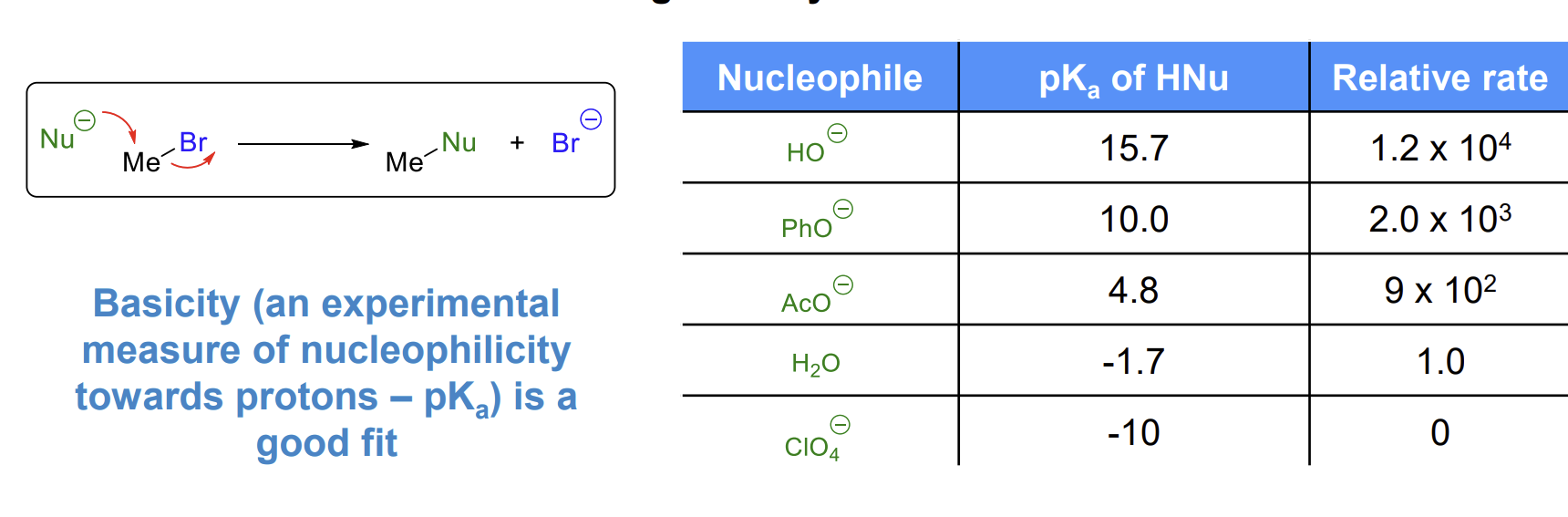
Increasing nucleophilicity towards a saturated carbon mirrors increasing basicity
Hence pKa and nucleophilicity are related but only for the same nucleophilic atom (oxygen in this case)
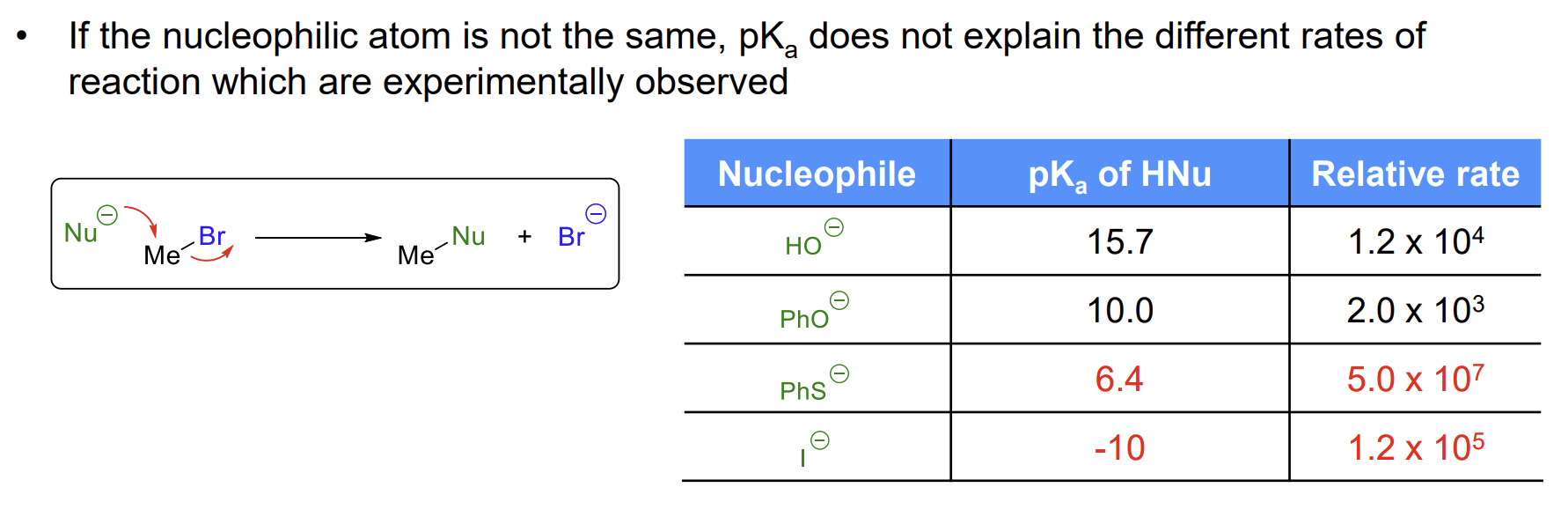
Can pKa be used to compare nucleophilicity across different atoms
No
How does nucleophilicity change down the group and why
Increases down the group
Larger atoms are more polarisable as their valence electron orbitals are more diffuse - small atoms are hard, large atoms are soft.
The HOMOs of large, soft atoms are higher in energy, making it less energy-requiring to remove these electrons for donation.
Hard-hard and soft-soft reactions are favoured in general
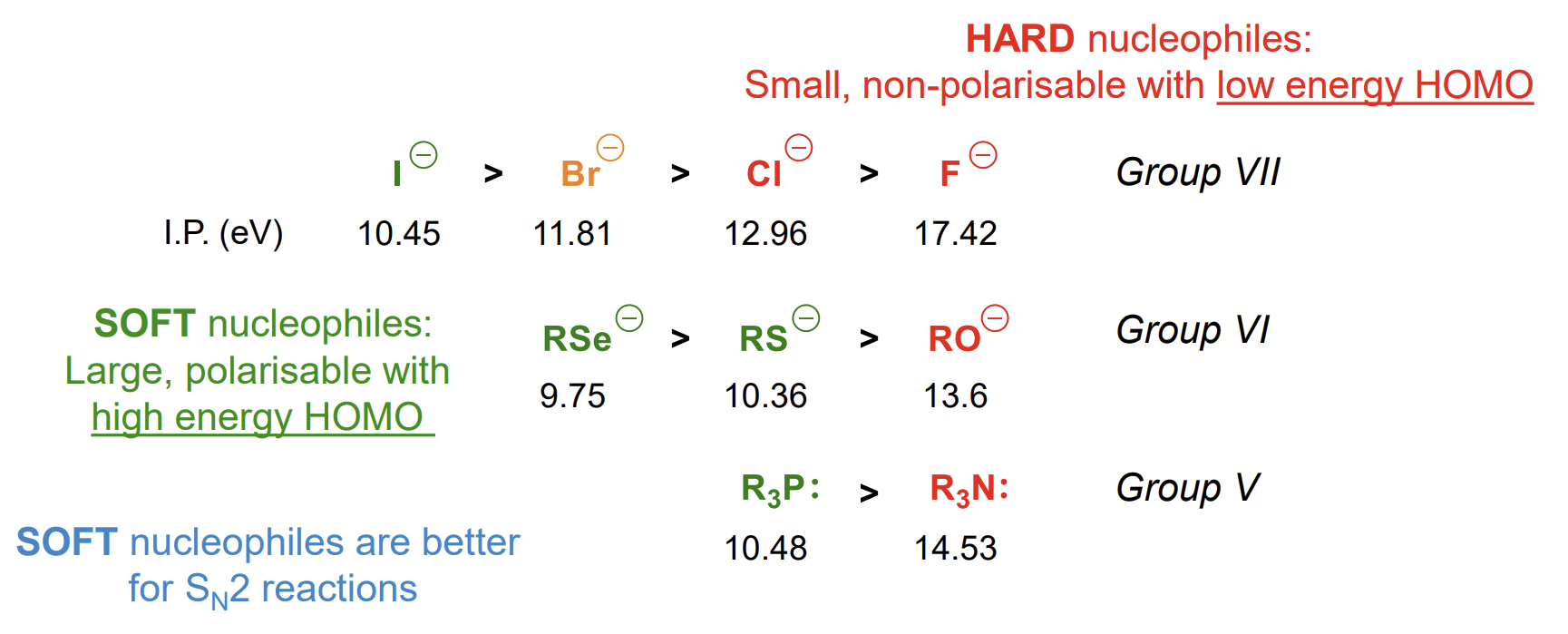
HOMO-LUMO interactions in SN2 for different elements
Energy gap is smaller for softer nucleophiles, increasing the rate of reaction
Stronger orbital overlap occurs when the HOMO and LUMO are similar in energy
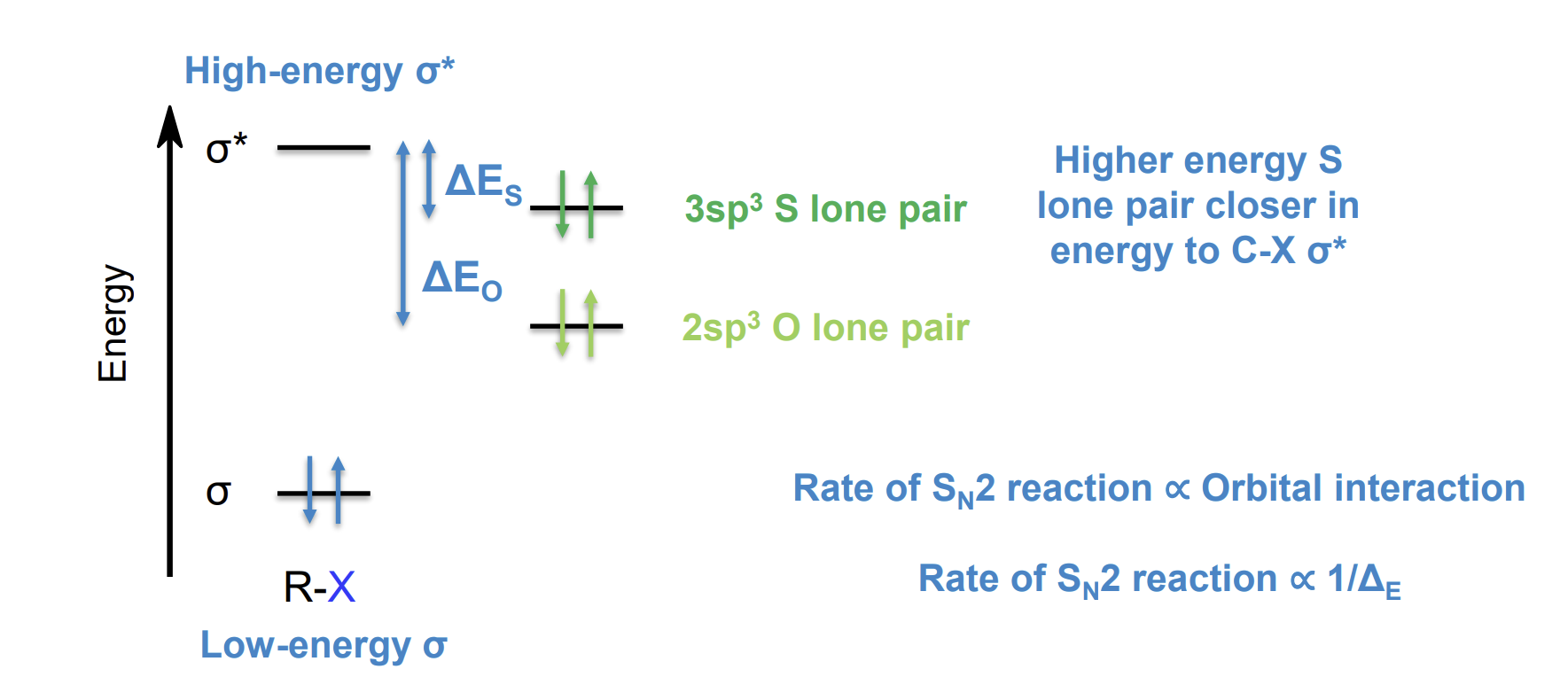
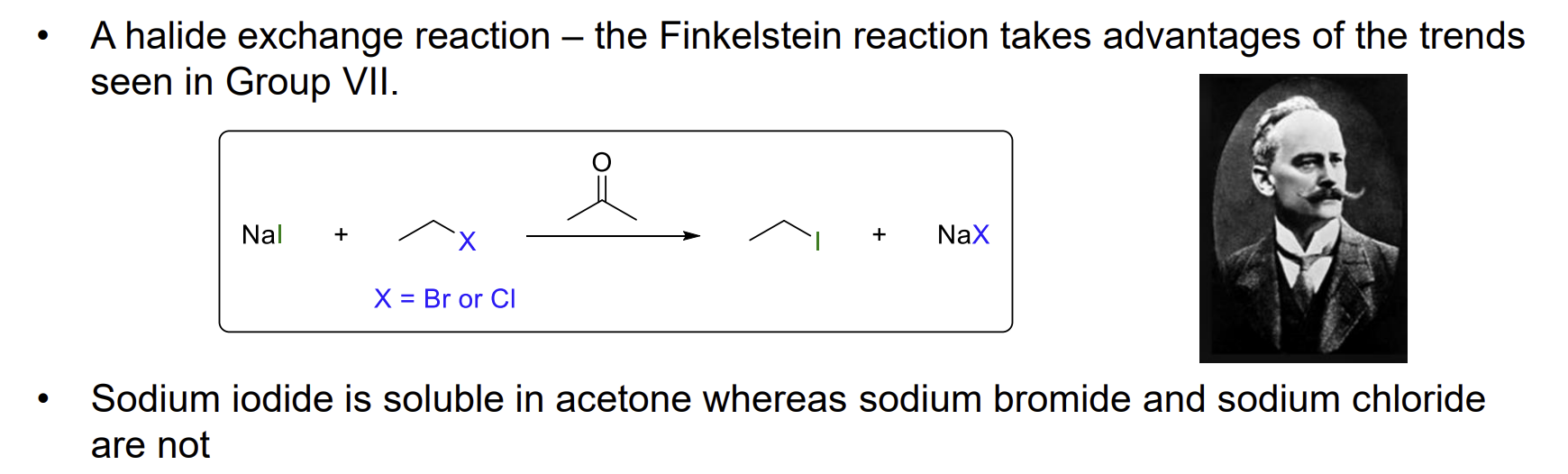
Finkelstein reaction - halide exchange
This reaction essentially allows NaI to act as a catalyst in some reactions as I is a better nucleophile than Cl and Br
Oxygen nucleophiles
Sulfur nucelopphiles
what is overalkylation
ammonia can react with alkyl halides multiple times, forming a mixture of primary to quaternary amines.
instead, azides can be used to form only one major product - a primary amine. This requires a hydrogenation step.
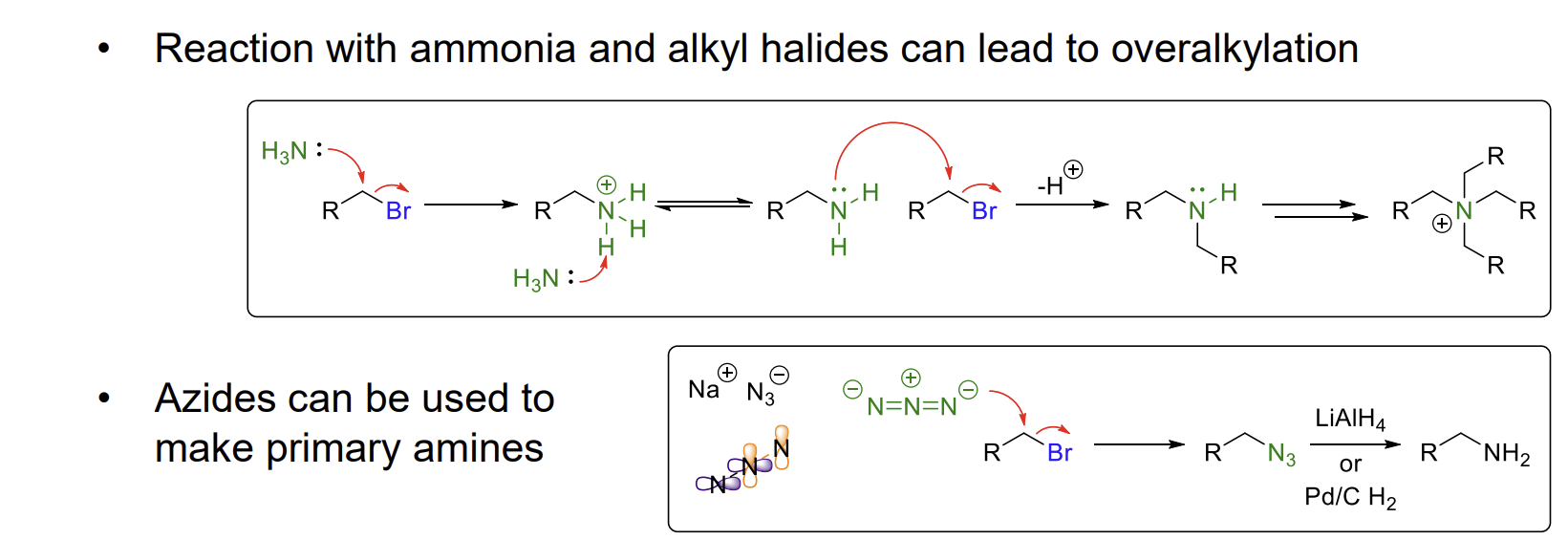
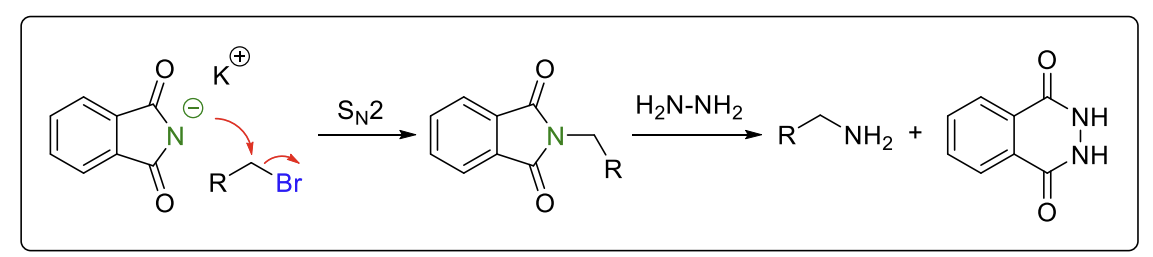
Gabriel synthesis
Method to produce primary amines
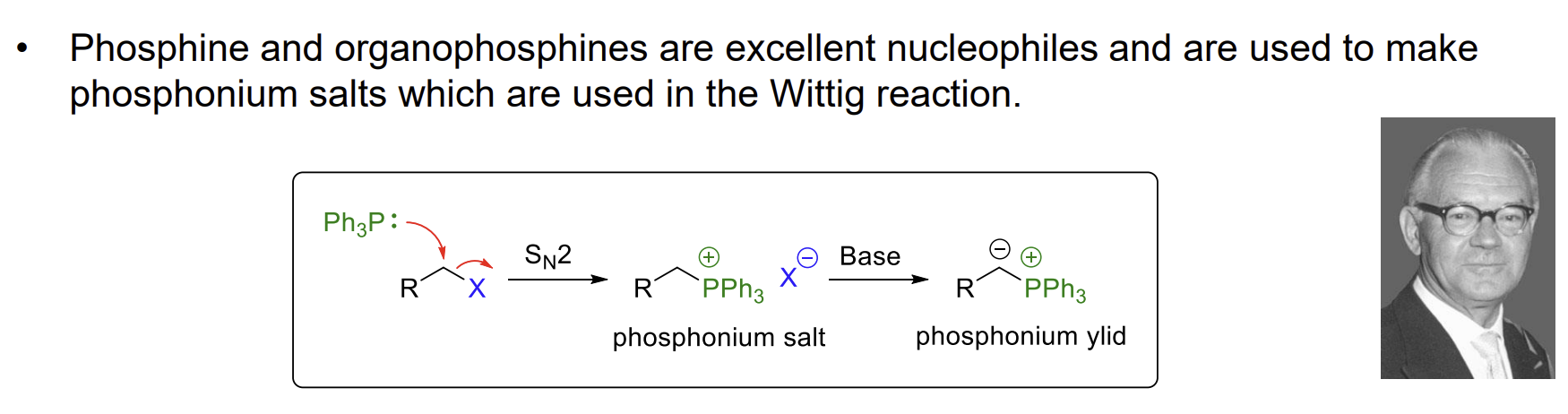
Phosphorus nucleophiles
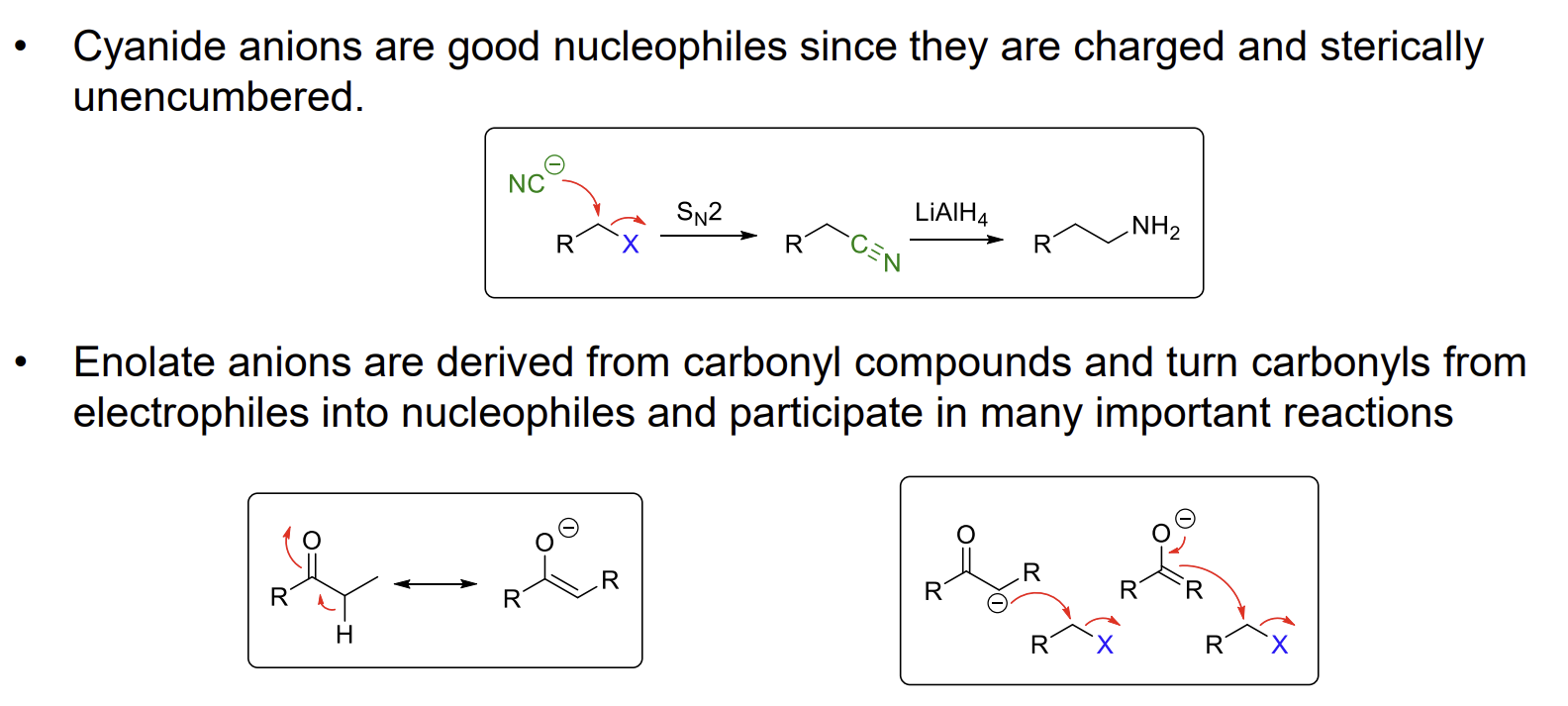
Carbon nucleophiles
Carbon is softer than N as its HOMO is higher in energy so the reaction proceeds from the carbon end. An important consideration about cyanide synthesis is that it adds a carbon atom to the chain after the reduction.
What happens if you increase the concentration of nucleophile in an SN1 reaction.
The rate of reaction will not increase however a competing elimination reaction may start occurring