additional practice problems
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
How does a change in the quaternary structure result in sickling of RBCs?
a. The formation of a hydrophobic pocket results in the B-globin molecules
polymerizing into fibers resulting in sickle-cell shapes
b. The formation of a hydrophilic pocket allows for an increase in O2 binding affinity
c. The formation of a hydrophobic pocket allows Glutamic acid to bind other
Glutamic acids, resulting in the sickling at the center of a sickle cell
d. The change in quaternary structure results in a mutation of the cell, causing it to
have an overall negative charge
a. The formation of a hydrophobic pocket results in the B-globin molecules
polymerizing into fibers resulting in sickle-cell shapes
Sickle Cell Anemia is caused by the codon encoding:
a. A deletion of glutamate
b. A point mutation resulting in a substitution of glutamate to valine
c. A missense mutation at amino acid 25
d. A point mutation resulting in a substitution of glutamate to histidine
b. A point mutation resulting in a substitution of glutamate to valine
What pair of values correctly describe someone who has Sickle Cell Disease?
a. Increased MCV, Iow HCT
b. Decreased MCV, normal HCT
c. Normal MCV, high HCT
d. Normal MCV, low HCT
d. Normal MCV, low HCT
What would you expect to see for your lab values in someone with an iron deficiency
anemia?
a. low MCV
b. high MCV
c. Normal MCV
a. low MCV
Where are enzymes normally found?
a. In the blood
b. Within cells
b. Within cells
What is the difference between plasma and serum?
a. Plasma requires you to add an anticoagulant before centrifugation
b. Serum requires you to add an anticoagulant before centrifugation
c. In plasma, you can observe a blood clot after you centrifuge
d. For plasma, you don’t have to do anything before centrifugation
a. Plasma requires you to add an anticoagulant before centrifugation
Which of the following tests would you order if you want to check someone’s liver
function?
a. CMP
b. CT scan
c. BMP
d. EKG
a. CMP
While gathering a patient's history, you discover that your patient likes to drink a six pack
of beer in the morning to “wake them up” and enjoys a bottle of scotch at night to “wind
down after a long day at work.” You find out this has been going on for the past 10 years.
Which of the following lab values would you expect to see on their LFTs?
a. Increase in ALP and GGT
b. AST to ALT in a 2:1 ratio
c. AST to CK in a 1:1 ratio
d. LDH and GGT elevated in a 3:1 ratio
b. AST to ALT in a 2:1 ratio
Classic finding in chronic alcohol abuse and alcoholic liver disease; AST is often about twice ALT, and both are elevated
Which isoenzyme of CPK is most specific for an MI?
a. CK-MM
b. CK-MB
c. CK-BB
b. CK-MB
CK-MM is mostly found in skeletal muscle, so it’s not specific for heart damage.
CK-MB is found primarily in cardiac muscle, making it the best marker for myocardial infarction (heart attack).
CK-BB is found mainly in the brain and smooth muscle, so it’s not useful for diagnosing MI.
A patient presents to the ER after having chest pain for the past 40 hours. What would
you expect your levels of CK-MB and LD-1 to be?
a. CK-MB would be elevated at its peak value, while LD-1 would still be within
normal range.
b. CK-MB would be low and almost back to its normal range, while LD-1 would be
elevated at its peak value.
c. Both CK-MB and LD-1 would be at their peak values since they would peak at
the same time.
d. Both CK-MB and LD-1 would be in normal range since these values don’t show
up until 48 hours after the onset of an MI
b. CK-MB would be low and almost back to its normal range, while LD-1 would be
elevated at its peak value.
Your lab results come back and all they tell you is that LDH is elevated, but you can’t tell
which isoenzymes are contributing to this elevation. What type of assay does this
describe?
a. Enzyme activity assay
b. Immunological activity assay
c. Electrophoretic separation
a. Enzyme activity assay
An enzyme activity assay measures the total activity of the enzyme (like LDH) without distinguishing which isoenzymes are present. To identify specific isoenzymes, you’d need methods like electrophoretic separation or immunological assays.
A four-year old boy presents to an urgent care clinic with severe vomiting that began two
days ago. If the vomiting continues, what pH imbalance would you predict might
develop?
a. Metabolic acidosis
b. Metabolic alkalosis
c. Respiratory acidosis
d. Respiratory alkalosis
Metabolic alkalosis
(A four-year old boy presents to an urgent care clinic with severe vomiting that began two
days ago) For the question above, what type of compensation will take place?
a. Metabolic compensation- retain more acids
b. Metabolic compensation- retain more bases
c. Respiratory compensation via hypoventilation
d. Respiratory compensation via hyperventilation
c. Respiratory compensation via hypoventilation
Severe vomiting causes loss of stomach acid → metabolic alkalosis → the body compensates by hypoventilating to retain CO2 (acid), lowering pH back toward normal.
(A four-year old boy presents to an urgent care clinic with severe vomiting that began two
days ago) For the question above, if this boy’s primary pH imbalance was fully compensated by the mechanism provided in your answer to question 30, what would you predict his blood pH to be?
a. 7.35
b. 7.40
c. 7.20
d. 7.45
d. 7.45
A 48-year old woman presents to the ER with difficulty breathing. She has smoked 2 packs a day for 30 years. How would you characterize this patient’s primary pH imbalance?
a. Metabolic acidosis
b. Metabolic alkalosis
c. Respiratory acidosis
d. Respiratory alkalosis
c. Respiratory acidosis
A patient presenting with an increased respiratory rate will often be associated with pO2 levels:
a. Above reference range
b. Below reference range
c. Within reference range
a. Above reference range
An unconscious patient has blood gas data: pH = 7.20 (7.35-7.45), pCO2 = 29 mm Hg (35-45), HCO3- = 9 mM (22-26). What primary pH imbalance exists?
a. Metabolic acidosis
b. Metabolic alkalosis
c. Respiratory acidosis
d. Respiratory alkalosis
a. Metabolic acidosis
Q: Which of the following would be an accurate reason for someone having a macrocytic anemia?
a. Vitamin B12 deficiency
b. Folate deficiency
c. Iron deficiency
d. Both A and B
e. Both B and C
d. Both A and B
Which of the following pairs of lab values correctly matches with its condition:
a. Iron deficiency- low MCV, low MCHC
b. folate/B12 deficiency- normal MCV, high RDW
c. Kidney failure- low MCV, high MCHC
d. Hemolytic- high MCHC, normal CR
a. Iron deficiency- low MCV, low MCHC
What symptoms could you see in someone with anemia?
a. Dizziness
b. Easily fatigued
c. Pallor of skin
d. All of the above
d. All of the above
In low O2 environments, what state would you expect hemoglobin to be in?
a. T state- deoxyhemoglobin
b. T state- oxyhemoglobin
c. R state- deoxyhemoglobin
d. R state- oxyhemoglobin
a. T state- deoxyhemoglobin
In low O2 environments, hemoglobin is mostly in the T state (deoxyhemoglobin) because it has released oxygen to the tissues and is in its low-affinity, tense form that favors oxygen release rather than binding
In high O2 environments, what state would you expect hemoglobin to be in and what
would its oxygen binding affinity look like?
a. R state- low oxygen binding affinity
b. R state- high oxygen binding affinity
c. T state- low oxygen binding affinity
d. T state- high oxygen binding affinity
b. R state- high oxygen binding affinity
In high O2 environments (like the lungs), hemoglobin is mostly in the R state (relaxed state), which has a high oxygen binding affinity to pick up oxygen efficiently.
Describe the difference between myoglobin and hemoglobin.
a. Myoglobin is used for oxygen transport
b. Hemoglobin is found in muscle
c. Myoglobin is used for oxygen storage
d. Hemoglobin is a monomer
c. Myoglobin is used for oxygen storage
How does the state of hemoglobin change in the lungs?
a. R to T state
b. T to R state
b. T to R state
How does the state of hemoglobin change in the tissues?
a. R to T state
b. T to R state
a. R to T state
The oxygen binding curve of hemoglobin can be described as:
a. Hyperbolic
b. Exponential
c. Sigmoidal
Sigmoidal
What does the Bohr effect explain?
a. Increased [H+] and pCO2 decrease O2 binding to hemoglobin due to Hb having
increased affinity for hydrogen than O2
b. Increased pH decreases O2 binding to hemoglobin
c. The stabilization of the T state favors O2 binding to hemoglobin
d. pH of the tissues and lungs do not affect the release of O2 from hemoglobin
a. Increased [H+] and pCO2 decrease O2 binding to hemoglobin due to Hb having
increased affinity for hydrogen than O2
Which of the following shifts the oxygen binding curve to the right?
a. High hydrogen ions (H+)
b. High 2,3-BPG
c. High pO2
d. Both A and B
d. Both A and B
Which of the following shifts the oxygen binding curve to the left?
a. Low pCO2
b. High pO2
c. Low pH
d. Both A and B
d. Both A and B
Which of the following amino acids would you expect to be phosphorylated by kinase
enzymes?
a. Glycine
b. Serine
c. Tyrosine
d. Both A and B
e. Both B and C
e. Both B and C
Kinase enzymes phosphorylate amino acids with -OH (hydroxyl) groups on their side chains.
Glycine (A) does not have a side chain with a hydroxyl group, so it is not phosphorylated.
Why are disulfide bonds important when building a protein?
a. They reduce electrostatic repulsion
b. They maintain stability and prevent denaturation
c. They have low steric hindrance
d. They increase the hydrogen bonding available to the protein
b. They maintain stability and prevent denaturation
A researcher is looking at the structure of a protein of interest. They notice that the
hydrogen bonds run parallel to the axis. Which of the following protein structures would
this be expected to be seen in?
a. Primary structure
b. Alpha helix
c. Beta sheet
d. Tertiary structure
b. Alpha helix
What would be the effect of someone with a mutation in their heat shock proteins that
prevent these proteins from working properly?
a. Nothing- heat shock proteins are not essential
b. The patient would be more easily fatigued
c. Cell toxicity due to improper folding and alignment
c. Cell toxicity due to improper folding and alignment
Which of the following correctly describes the properties of an enzyme?
a. Not consumed during a reaction
b. Increase the amount of product in a given reaction
c. Increase the rate of a given reaction
d. Both A and C
d. Both A and C
How do temperature and pH influence velocity of an enzyme?
a. Enzymes function within an optimal temperature and pH range- anything too
outside of that range will denature the enzyme
b. Only pH affects the velocity of the enzyme since an extremely high or low pH can
denature an enzyme
c. All enzymes can function at all temperatures but have a specific pH that they will
only work in
d. Only temperature affects velocity of an enzymatic reaction since enzymes can
function at any pH
a. Enzymes function within an optimal temperature and pH range- anything too
An enzyme with a high affinity for its substrate will exhibit:
a. High Km
b. Increased [substrate] needed to reach Vmax
c. Low Km
c. Low Km
Lead poisoning is an example of:
a. Reversible binding
b. Uncompetitive inhibition
c. Irreversible binding
d. Competitive inhibition
c. Irreversible binding
Statin drugs work by:
a. Increasing the producing of HMG CoA reductase
b. Inhibiting HMG CoA reductase competitively
c. Causing the breakdown of mevalonic acid
d. Increasing the production of HMG CoA
b. Inhibiting HMG CoA reductase competitively
Which of the following correctly describe a non-competitive inhibitor:
a. Km= unchanged, Vmax= increased
b. Km= increased, Vmax= unchanged
c. Km= unchanged, Vmax= decreased
d. Km= decreased, Vmax= unchanged
c. Km= unchanged, Vmax= decreased
Why does hemoglobin exhibit a sigmoidal oxygen-dissociation curve, whereas
myoglobin exhibits a hyperbolic one?
a. Hemoglobin has multiple heme groups and shows cooperative binding, while
myoglobin binds one oxygen molecule without cooperativity
b. Myoglobin is found in red blood cells, while hemoglobin is found in muscle
c. Hemoglobin binds oxygen with lower affinity than myoglobin at all pO₂ levels
d. Myoglobin binds to 2,3-BPG, whereas hemoglobin does not
a. Hemoglobin has multiple heme groups and shows cooperative binding, while
myoglobin binds one oxygen molecule without cooperativity
Carbon monoxide impairs oxygen delivery by hemoglobin primarily because:
a. Carbon monoxide irreversibly binds hemoglobin.
b. Carbon monoxide causes hemoglobin to denature.
c. Carbon monoxide shifts hemoglobin into the R state and increases oxygen
affinity, preventing release to tissues.
d. Carbon monoxide binds only to myoglobin, displacing oxygen.
c. Carbon monoxide shifts hemoglobin into the R state and increases oxygen
affinity, preventing release to tissues.
42. In a patient experiencing anemia, you note that their corrected reticulocyte value is
under 2.5%. What does this tell you about this patient?
a. The body is responding appropriately to the anemia and re establishing
homeostasis
b. There is a deficient production of RBCs in the bone marrow, and the body is not
responding appropriately.
c. The corrected reticulocyte value does not tell us much and does not take into
account if the patient is anemic.
b. There is a deficient production of RBCs in the bone marrow, and the body is not
responding appropriately.
What is the difference between recombinant insulin and recombinant insulin glargine?
a. Recombinant insulin is slow-acting due to its high electrostatic repulsion and
decreased solubility.
b. Recombinant insulin is fast-acting due to its hydrophobicity and low electrostatic
repulsion.
c. Recombinant insulin glargine has the addition of Glycine and 2 Arginines, lending
to its hydrophobicity and low electrostatic repulsion, making it a slow-acting drug.
d. Recombinant insulin glargine has the addition of Glycine and 2 Arginines, making
it hydrophilic and highly repulsive, making it slow-acting.
c. Recombinant insulin glargine has the addition of Glycine and 2 Arginines, lending
to its hydrophobicity and low electrostatic repulsion, making it a slow-acting drug.
The Ka of hydroiodic acid (HI) is 3.16 X 109 and for acetic acid it is 1.75 X 10-5. Which of the
following best describes their comparative strength in acidity?
a. Hydroiodic acid is a stronger acid than acetic acid with more dissociation into H+.
b. Hydroiodic acid is a stronger acid than acetic acid with less dissociation into H+.
c. Hydroiodic acid is a weaker acid than acetic acid with less dissociation into H+.
d. Hydroiodic acid is a weaker acid than acetic acid with more dissociation into H+.
a. Hydroiodic acid is a stronger acid than acetic acid with more dissociation into H+.
A patient is determined to have a metabolic acidosis. How would the body begin to
compensate for this pH imbalance?
a. Decrease breathing rate
b. Increase breathing rate
c. Decrease bicarbonate elimination
d. Increase bicarbonate elimination
b. Increase breathing rate
A patient has the following blood gas data. What is the pH imbalance?
pH = 7.12 (7.35-7.45)
pCO2 = 78 mmHG (35-45 mmHG)
pO2 = 75 mmHG (80-100 mmHG)
HCO3- = 28 mM (22-26 mM)
a. Metabolic acidosis
b. Metabolic alkalosis
c. Respiratory acidosis
d. Respiratory alkalosis
c. Respiratory acidosis
A patient has the following blood gas data.
pH = 7.12 (7.35-7.45)
pCO2 = 78 mmHG (35-45 mmHG)
pO2 = 75 mmHG (80-100 mmHG)
HCO3- = 28 mM (22-26 mM)
What is the compensation status for the patient in question 3 above?
a. Compensated
b. Overcompensated
c. Partially compensated
d. Uncompensated
c. Partially compensated
1. Which of the following amino acids is most likely to be on the exterior of a protein within the lipid
bilayer of a cell’s plasma membrane.
A. Glutamic acid
B. Cysteine
C. Isoleucine
D. Lysine
C. Isoleucine
2. The amino acid that is most likely to have a phosphate group removed from its R-group side chain
by a phosphatase enzyme is
A. Tryptophan
B. Serine
C. Proline
D. Arginine
B. Serine
3. An alpha-globin polypeptide subunit has quaternary structure.
A. TRUE
B. FALSE
TRUE
4. Hydrogen bond formation occurs perpendicular to the axis of a
A. Alpha-helix.
B. Helix-loop-helix motif.
C. Beta-bend.
D. Beta-sheet.
beta sheet
5. A recombinant protein can be made to dissolve more slowly in an aqueous environment by
increasing the net positive charge through the addition of a/an
A. lysine residue.
B. aspartate residue.
C. glycine residue.
D. threonine residue.
lysine residue
A change in which of the following may affect the rate of an enzyme-catalyzed reaction?
A. Reaction temperature
B. Reaction pH
C. Substrate concentration (when substrate concentration < Km)
D. ALL OF THE ABOVE
D all of the above
If a noncompetitive inhibitor is included in a reaction of enzyme and substrate,
A. the inhibition will be overcome if enough substrate is added.
B. inhibitor and substrate will bind to different sites on the enzyme.
C. Km will be decreased owing to reduced enzyme-substrate affinity.
D. the inhibitor will bind to the enzyme active site.
E. Vmax will remain the same as in the uninhibited reaction.
B inhibitor and substrate will bind to different sites on the enzyme.
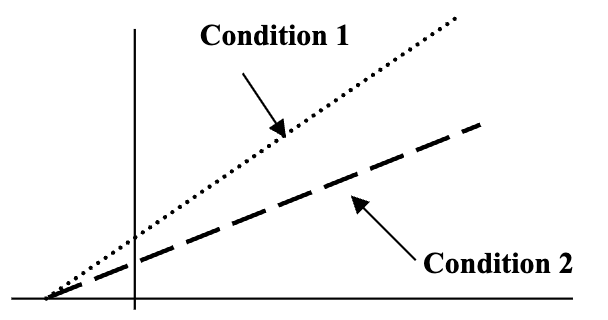
The graph shown depicts a Lineweaver-Burk
plot of an enzyme-catalyzed reaction under
two conditions, Condition 1 and Condition 2.
Which of the following statements best
describes the relationships between the
conditions depicted in the graph?
A. Condition 1 is a reaction with a competitive inhibitor and Condition 2 without one.
B. The same Vmax is observed for the reactions under both Condition 1 and Condition 2.
C. The Km in Condition 1 is a greater value than the Km observed in Condition 2.
D. In Condition 1 an inhibitor is probably binding a site other than the active site.
E. Addition of excess substrate will overcome the inhibition present in Condition 2
D. In Condition 1 an inhibitor is probably binding a site other than the active site.
In an enzyme-catalyzed reaction, Enzyme X is the substrate for Enzyme Y, a kinase. The
product of this reaction is
A. ATP
B. dephosphorylated Enzyme X
C. phosphorylated Enzyme X
D. dephosphorylated Enzyme Y
E. phosphorylated Enzyme Y
C. phosphorylated Enzyme X
Sulfa drugs are effective in limiting bacterial infections while not producing toxic effects in
human cells. To account for these characteristics, sulfa drugs most likely act as a(n)
A. noncompetitive inhibitors of several regulatory steps in glycolysis.
B. competitive inhibitor of an enzyme that is unique to mammals.
C. positive allosteric effector of an enzyme in bacterial cell wall synthesis.
D. inhibitor that interrupts replication in all types of dividing cells.
E. competitive inhibitor of an enzyme required only by bacteria.
E. competitive inhibitor of an enzyme required only by bacteria.
1. Increased blood plasma levels of ALT (SGPT) will most likely result from
A. cell death in the pancreas.
B. hemolytic anemia.
C. liver damage.
D. increased muscle mass.
E. intense physical exercise.
C. liver damage.
2. Creatine kinase is an enzyme whose normal function occurs predominantly within
A. blood.
B. erythrocytes.
C. liver.
D. muscle.
E. pancreas.
D. muscle.
3. Detection of the LD5 (MMMM) isoenzyme of lactate dehydrogenase in serum from a patient
A. is accomplished by an enzyme assay to measure total LDH activity.
B. is characteristic of a recent myocardial infarction.
C. may result from increased renal clearance and not from pathology.
D. reveals the presence of excess substrate for the enzyme within the blood.
E. would be done using either electrophoresis or an immunological activity assay.
E. would be done using either electrophoresis or an immunological activity assay.
4. Which of the following isoenzyme patterns is observed soonest after a myocardial
infarction?
A. HIGH CPK (total) and INCREASED LD5
B. INCREASED CK-BB and Normal LD5
C. INCREASED CK-MB and INCREASED LD1
D. INCREASED CK-MB and Normal LD1
E. Normal CPK (total) and INCREASED LD1
D. INCREASED CK-MB and Normal LD1
5. A healthy 25-year-old man moves from Boston to Denver, the “mile high city.” A few months later, which of the
following would be expected to have increased in his red blood cells compared to when he lived at sea level?
A. 2,3-BPG
B. CO2
C. Hemoglobin
D. H+
A. 2,3-BPG
1. The oxygen dissociation curve for myoglobin indicates that myoglobin
A. has subunits that cooperate in binding oxygen.
B. reversibly binds a single molecule of oxygen.
C. responds to small changes in pO2 in tissues.
D. binds oxygen in lungs and releases it in tissues.
B. reversibly binds a single molecule of oxygen.
2. The T or “taut” confirmation of hemoglobin is characterized by
A. Low affinity for oxygen
B. High affinity for oxygen
C. Half of its binding sites occupied with oxygen
D. Ruptured polar bonds between alpha beta dimers
A. Low affinity for oxygen
3. A usually healthy 24-year-old woman is seen by her family physician for an annual physical exam. She reports
feeling somewhat tired lately but otherwise has no health issues. Conjunctival pallor is observed when she is
examined. Which test result will best confirm the suspected diagnosis?
A. Low blood pH
B. Low hemoglobin
C. High blood level of iron
D. Low blood level of CO
B. Low hemoglobin
4. Which of the molecules below is an allosteric effector of hemoglobin that increases the oxygen affinity of
hemoglobin?
A. H+
B. Oxygen
C. 2,3-BPG
D. CO2
B. Oxygen