2.4 Crystal Structure
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
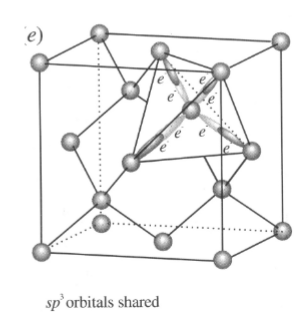
Covalent Bonded Minerals
-atoms need to be in specific arrangements
-orbitals must overlap
required to share electrons
prevents close packing
typically hard and high melting points
Diamond:
-electrons shared: highly oriented bonds; directional
-atom locations controlled by the orientation of the bonds
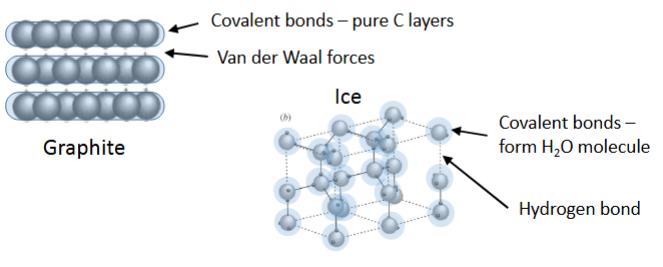
molecularly bonded crystals
Discrete molecules packed in systematic way
-molecules bonded internally with covalent/ionic bonds (single unit)
-molecules held together with an der waals or hyrdogran bonds
-example- graphite and ice
metallic bonded crystals
Atoms are all same size
atoms pack in regular arrangement. try to minimize open space in structure
three types of metallic packing
Hexagonal closest packing- hexagonal symmetry
cubic closest packing- cubic symmetry, 2 types:
-face centered
-body centered
Based on layering of spheres
Hexagonal Closest packing
-every 3rd layer is aligned vertically
-means each atom has 12 nearest neighbors
6 in layer and 3 above and below layer
-AB-AB-AB stacking
-symmetry is hexagonal, layers on [001] plane
![<p>-every 3rd layer is aligned vertically</p><p>-means each atom has 12 nearest neighbors</p><p>6 in layer and 3 above and below layer</p><p>-AB-AB-AB stacking</p><p>-symmetry is hexagonal, layers on [001] plane</p>](https://knowt-user-attachments.s3.amazonaws.com/6c1dcfb7-6525-41b3-82a1-4849dab6999d.png)
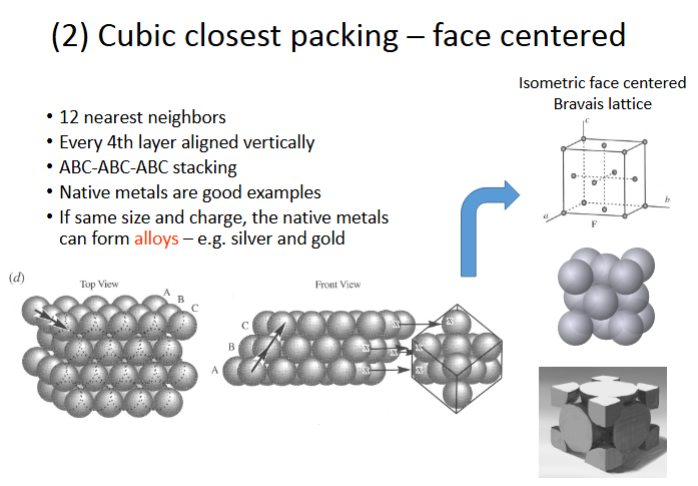
Cubic closest packing – face centered
-12 nearest neighbors
-every 4th layer aligned vertically
-ABC-ABC-ABC stacking
-native metals are good examples
-if same size and charge, the native metals can form alloys- ex. silver and gold
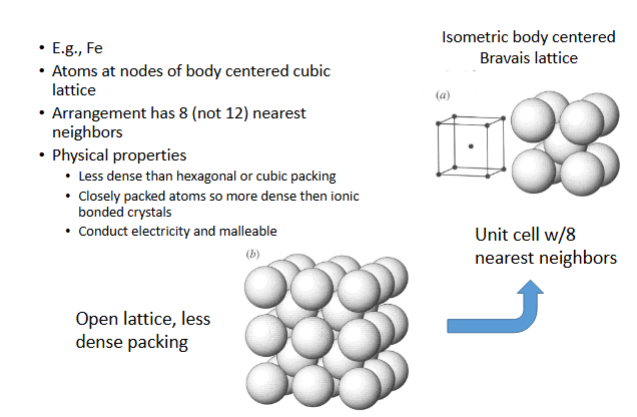
Cubic closest packing - Body-centered
Atoms at nodes of body centered cubic lattice
arrangement has 8 (not 12) nearest neighbors
physical properties:
-less dense than hexagonal or cubic packing
-closely packed atoms so more dense then ionic bonded crystals
-conduct electricity and malleable
Ionic Bonding
Oxygen most abundant anion making up earth
• Oxygen is highly electronegative = 3.5
Oxygen bonds mostly ionic
• Bonding characteristic of Si-O is 50% ionic
• Other elements (Al, Fe, Mg, Ca, Na, K) are even higher percentages ionic
bonds
If we assume 100% ionic bonds determine crystal structure, then:
• No directionality of bonds
• Does not require overlapping orbitals
• Only consider geometry (e.g. packing) of atoms
Structures similar to metallic bonds, but now different size and
charges spheres
Pauling’s Rules
• A set of rules to describe how ionic spheres can pack given assumptions
• Five rules:
1. Coordination Principle
2. Electrostatic Valency Principle
3. Sharing of Polyhedral elements I
4. Sharing of Polyhedral elements II
5. Principle of Parsimony
Coordination Principle (rule 1)
Coordination number
-number of ions surrounding central ion
-usually cation surrounded by anions
-common numbers are 12,8,6,4,3,2
-there can be others- 11,10… ect
Coordination polyhedron
-shape of the coordination complex
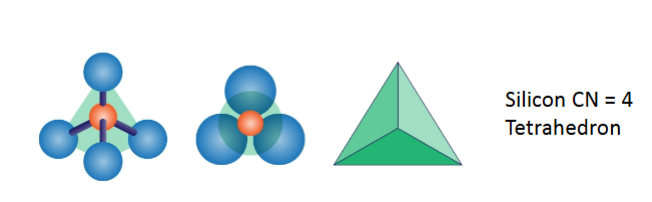
Coordination Polyhedron
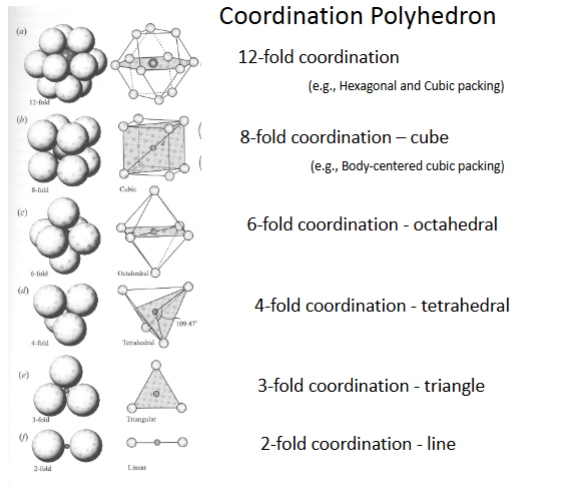
Coordination number
coordination number depends on radius ratio, RR:
RR=R cation/ R anion
Electrostatic valency principle (rule 2)
Bonding capacity is proportional to:
• Oxidation state (charge)
• Coordination number
• Quantified as electrostatic valence bond (evb)
evb = ion charge/CN
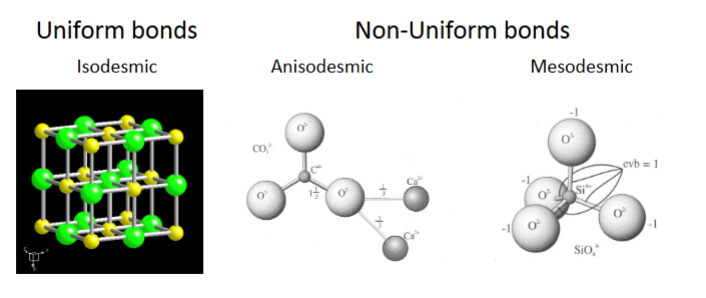
Two types:
• Uniform bond strengths - Isodesmic
• Non-uniform bond strengths – Anisodesmic & Mesodesmic
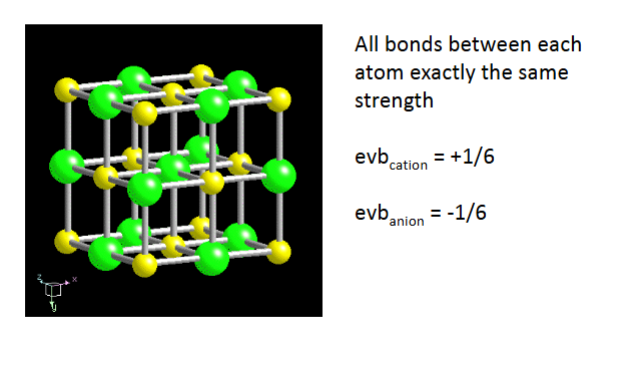
Isodesmic – Uniform bond strength
Isodesmic = equal strength
all ions the same charge
all bonds between cations and anions have same strength
anions tend to pack into highly symmetrical arrangement
-typical isometric, tetragonal hexagonal
-typically oxides, fluorides, chlorides
Non-uniform bond strength
-some bonds are stronger (ie greater evb) than others within an individual mineral
-forms anionic groups
-commonly oxygen with small, high charge cations
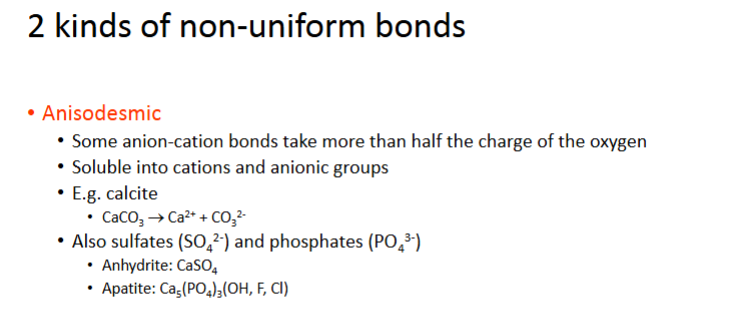
2 kinds of non-uniform bonds
Anisodesmic
Some anion-cation bonds take more than hlaf the charge of the oxygrn
soluble into cations and anionic groups
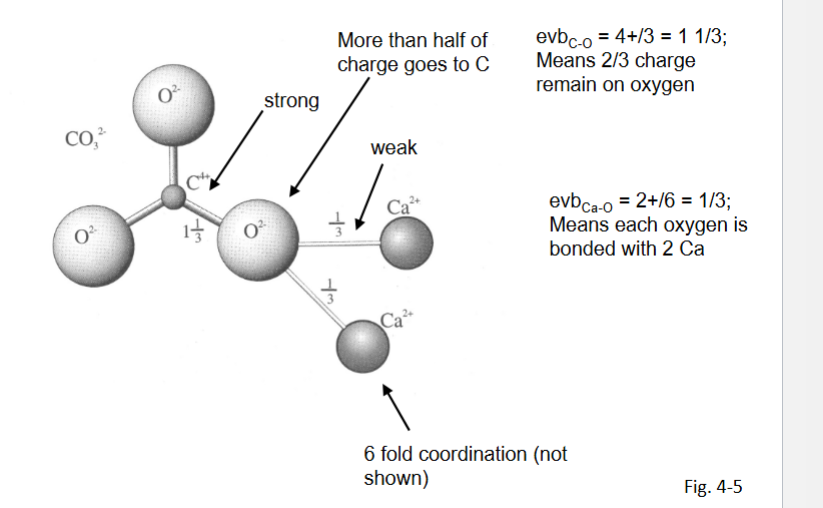
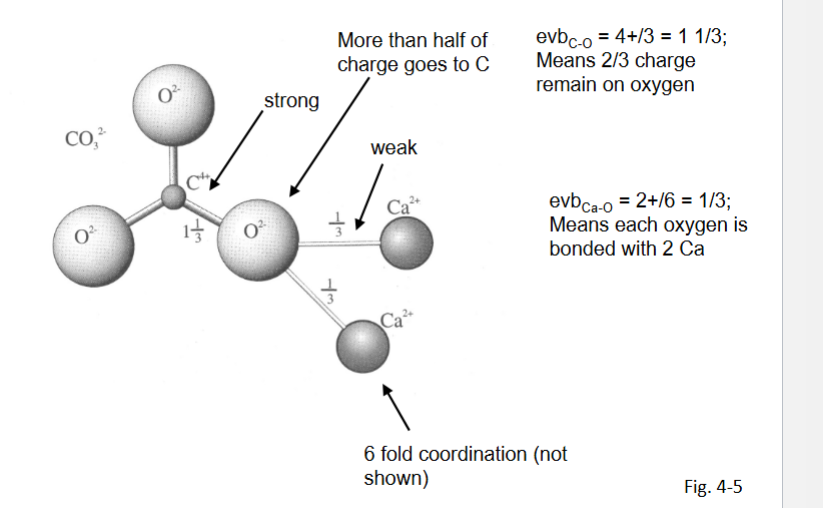
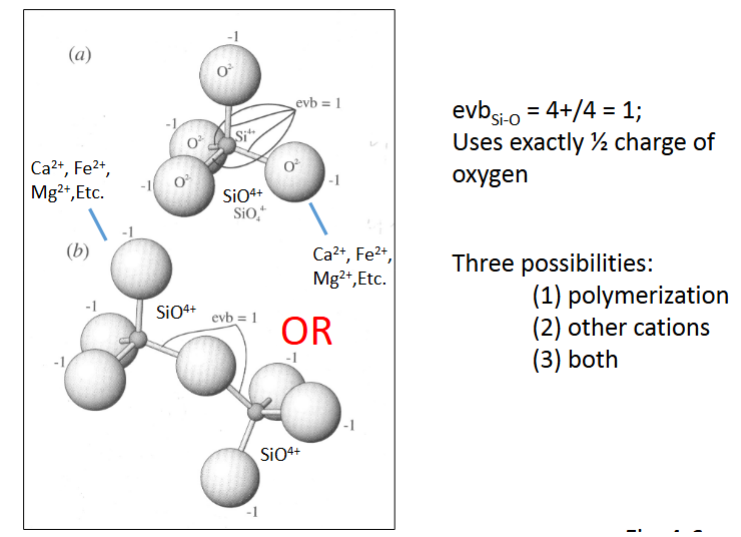
Mesodesmic
• Unlike isodesmic – charges on ions differ
• Nonetheless, anion-cation bonds take exactly half of the anion charge – e.g.,
SiO44-
• Silica bonds with 4 oxygen
• Silica tetrahedron
Mesodesmic minerals may bond with other ions
Silicates
• Quartz: SiO2 isodesmic-like bonding, equal bond strength throughout
• Olivine: (Fe,Mg)2SiO4 no silica tetrahedron bonded to other silica
tetrahedron.
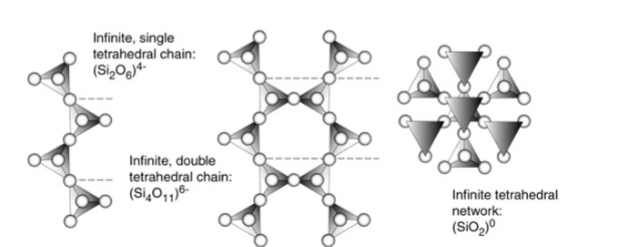
Mesodesmic bonding of silicates very important:
• Allows polymerization of silicate ion
• Arrangement of tetrahedron is basis of silicate mineral classification
Summary - evb
• evb = charge on cation/cation CN
• For any anion (usually O2-); Sum of all EVBs on anion = charge on
anion
• Required for electrical neutrality
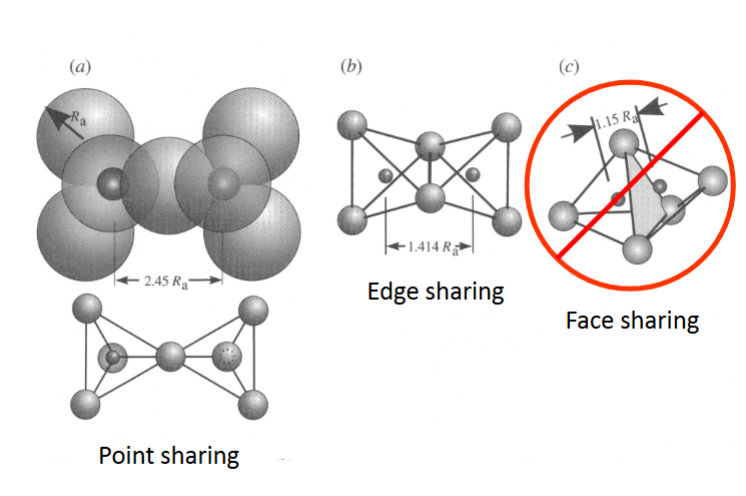
Sharing of polyhedral elements I
(rule 3)
• Cations generally share only single anions
point sharing
• Occasionally will share two anions
Edge sharing
• Never share 3 anions
Face sharing
• Reason for this is that cations have to be separated by sufficient
distance
Sharing of polyhedral elements II (rule 4)
• Highly charged cations are not placed near each other in a
structure
• Like charge repel – highly charged ions must be far apart
• Small cations (highly charge) have low coordination number
Use more than ½ of anion charge
E.g. CO32-, PO43-, SO44-,
• Other cations bonded to anions have low charge and are large
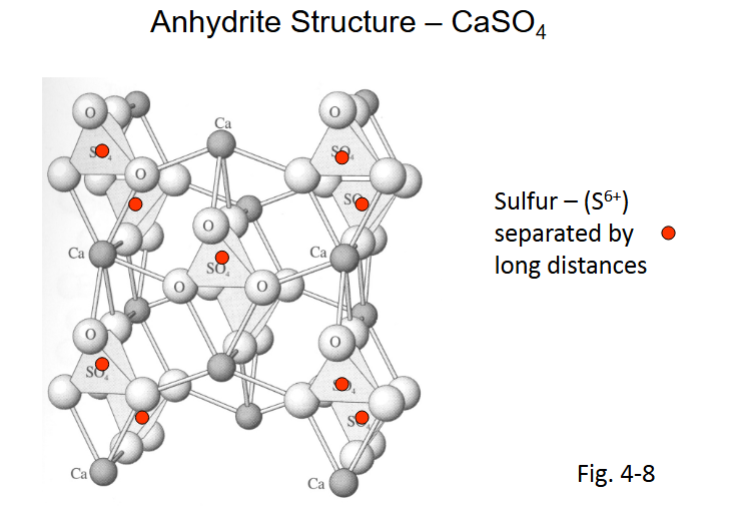
Principle of Parsimony
(rule 5)
• Parsimony = “stinginess”
• Number of fundamentally different sites for a mineral is small
• Typically fewer than 4 different coordination polyhedron (sites) for cations
• Means there are small integer ratios of elements in mineral formulas