Nutrition 4 [Controlling energy balance]
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Homoeostatic mechanisms contributes to energy balance
Energy stores
Obesity
Ob + db genes
Obesity and evolution
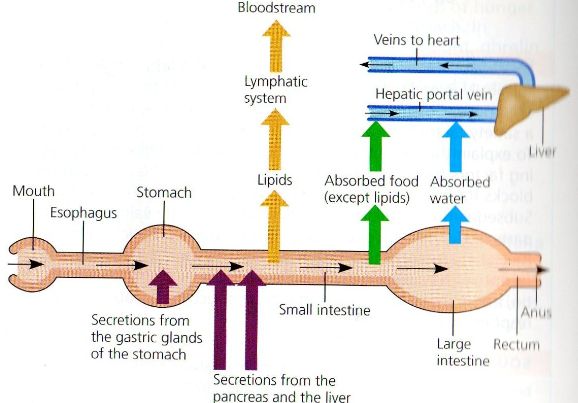
Overview of food processing in mammalian digestive tract
Energy balance
ATP
produces from oxidation of carbohydrates, proteins and fats in cellular respiration.
Energy stores
Preference of use = carbs + fats then proteins
Fats 2x energy released per gram cf. others
Excess molecules are converted to storage molecules:
1st site → glycogen
2nd site → adipose (fat) cells
glycogen
a polymer glucose, stored in liver and muscle. Tight regulation of glycogen levels
Adipose cells
Store energy as fat when glycogen stores full
Energy released diagram
Obesity
Overnourishment (consuming too many calories) causes obesity, the excessive accumulation of fat
Obesity problems that causes health problems
Type 2 diabetes
Cardiovascular disease
Colon cancer
Breast cancer
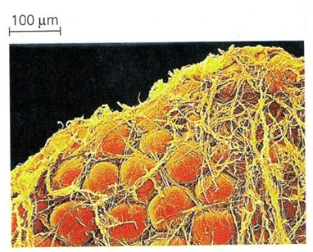
Fat cells from the abdomen of a human
Strands of connective tissue, shown in yellow, hold the fat storing adipose cells, shown in red in place.
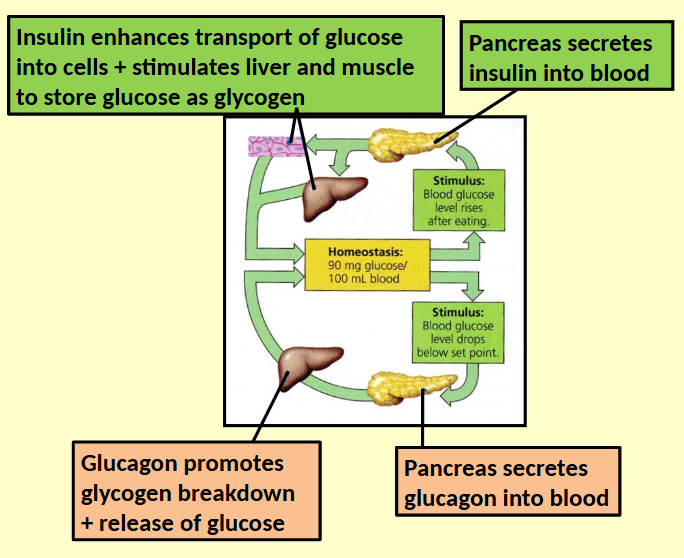
Body weight is regulated by homeostatic mechanism
Feedback circuits control storage and metabolism of fat
Hormones regulate ‘‘Satiety centre’’ in the brain
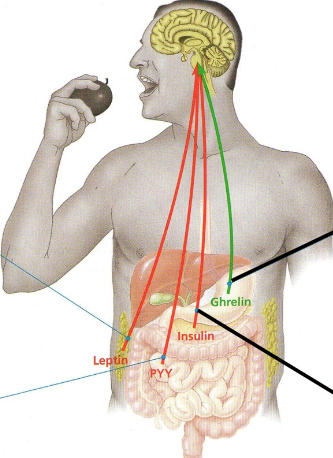
Appetite-regulating hormones
Secreted by various organs, hormones reach brain via bloodstream..
Green → appetite stimulant
Red → appetite suppressant
Ghrelin
Secreted by stomach wall
Triggers hunger at meal times
(increases in dieters, hence difficult to stay on diet!)
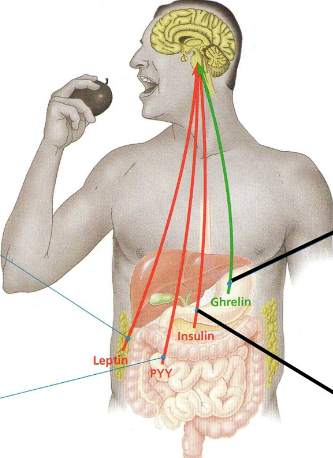
Insulin
Produced by pancreas after rise in blood sugar
As well as other functions also suppresses appetite
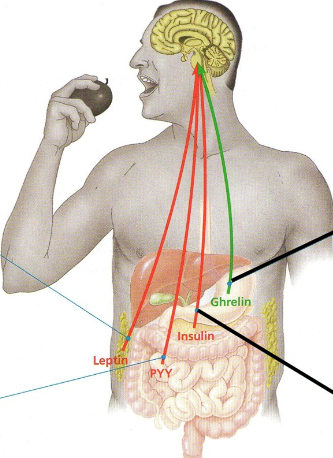
Leptin
Produced by adipose tissue and suppresses appetite
Decrease in body fat causes levels to fall, hence appetite increases.
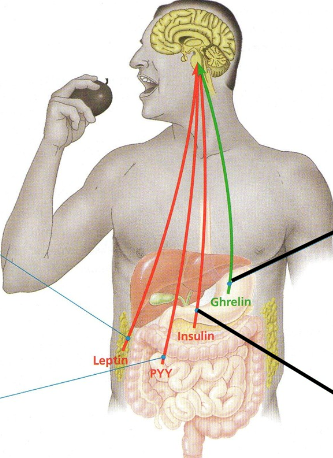
PYY
Secreted by small intestine after meals
Appetite suppressant that counters effect of ghrelin
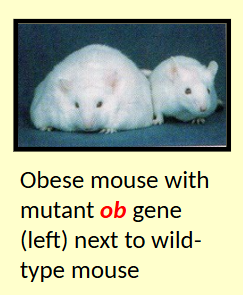
ob + db genes in appetite regulation
Mutations in chronically obese mice investigated
Mutation in ob gene leads to mouse eating voraciously = obese
ob gene required to produce satiety factor
db gene required to respond to that factor
ob gene actually encodes for a hor
Obesity + Evolution
Hunter gatherer ancestors → seeds and plants, with occasional meat from hunting ‘‘feast or famine’’.
Promotes individual with high capacity for storing = more likely to survive lean times
Offspring of petrel
Parents on long food trips
Food has high fat content but little protein
Chicks have to ‘‘overfeed’’ to get enough protein
Can survive while parents are away
Eventually need to fast for several days to lose weight to be able to fly

ob genes
ob gene required to produce satiety factor
the gene encodes for a hormone, leptin

db genes
db gene required to respond to that factor
the gene encodes for leptin receptor

Mammalian hibernation
Physiological and morphological changes to survival seasonal low temperatures and food scarcity
Prolonged bouts of ‘‘torpor’’ save energy, body temp falls (to 0-10*C)
Reduced metabolic rate (-5% of active levels)
13-lined ground squirrel
consumes no food for 5-6 months
Rely on fat stores for energy
Changes in gastrointestinal tract particularly noticeable.

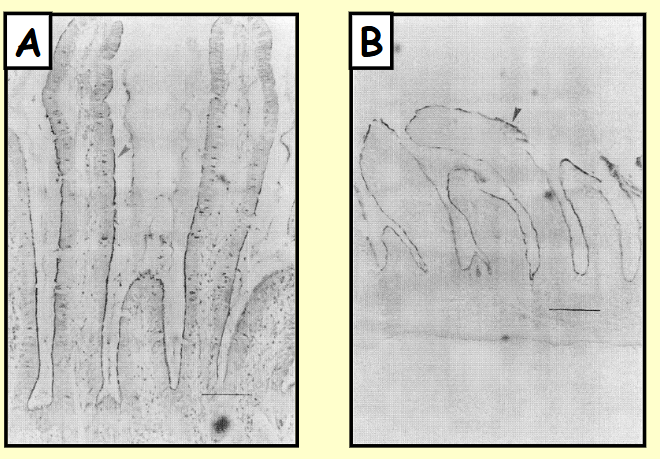
Ground squirrel jejunum
Stained with antibody that detects brush border enzyme sucrase-isomaltase (marked by arrows)
(A) active animal (B) hibernating / torpor animal
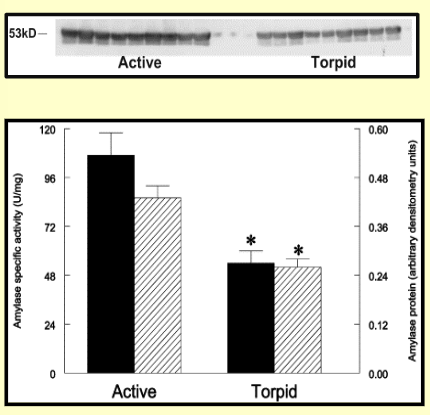
Hibernation reduces pancreatic amylase levels
Torpid → no food for 6 weeks
Amylase activity down 50%
Amylase protein expression down 40%
Remaining levels facilitate digestion upon awakening in spring
GI tract during hibernation, what happens?
During hibernation GI tract is reduced in size / weight, with reduction in number of cells = not needed for digestion
Preservation of intestinal gene expression during hibernation so still totally functional - no lag time in recovery upon awakening
Urea Nitrogen Salvaging & Hibernation
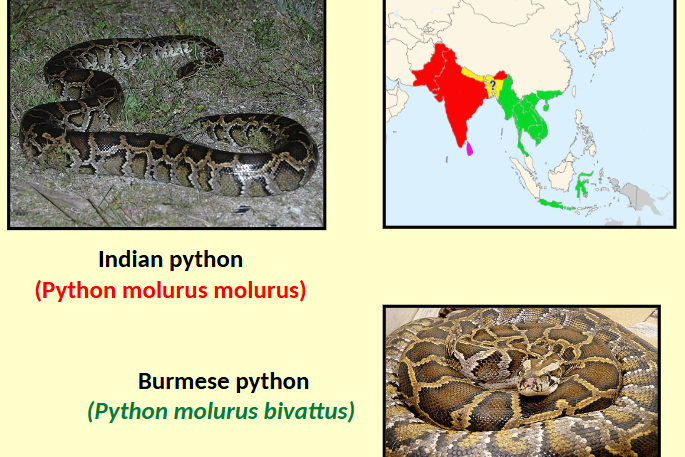
Indian and Burmese python
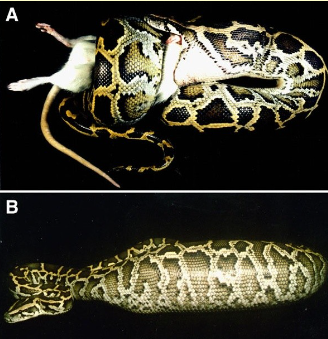
Burmese python digestion
Burmese python swallowing a rat, killed by constriction
24 hours after consuming a rat meal (>50% of snake’s body mass). Further distention of body due to the build up of gases within ingested rat).
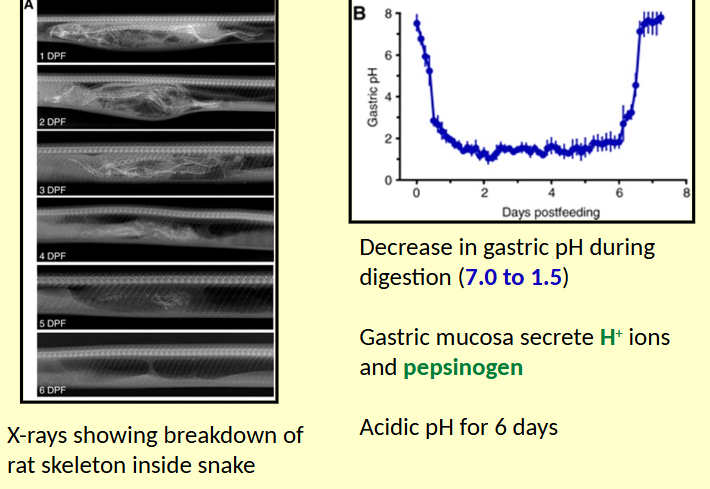
Python digestion
Pythons wait for suitable prey to wander near; may wait for weeks on end
Equipped to ingest large animals if give the chance (up to 70% of their own weight)
Within 24 hours of feeding, double the mass of mid-gut through growth of new epithelia.
20-fold increase in transport proteins (e.g. glucose and amino acid transporters)
Metabolic rate increases 40-fold
Python post feeding
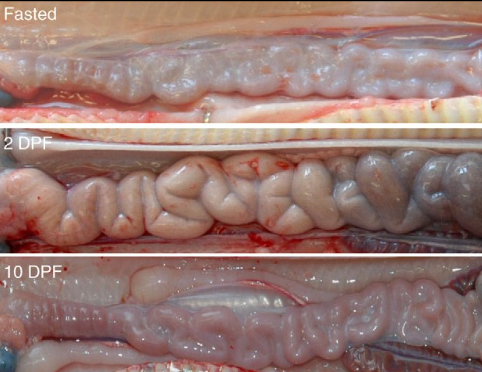
Small intestine of similar-size pythons fasted, or at 2 and 10 days postfeeding (DPF). By 2 DPF, intestine in diameter due to hypertrophy of the epithelial cells; response reversed by 10 DPF
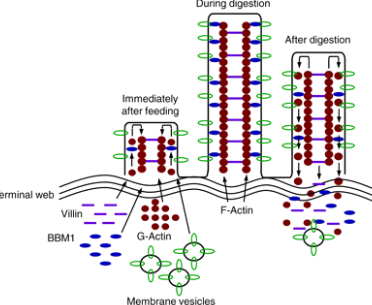
Model for lengthening of microvilli
begins after onset of feeding
involves structural proteins actin and villin
5-fold increase in villi and apical surface area during digestion
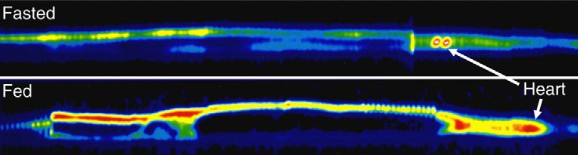
Positron emission tomography (PET) images of fasted and fed (1 DPF) Burmese python
injected with 2-[18F]fluoro-2-deoxyglucose
bright areas = regions experiencing high rates of glucose metabolism
Difference between images is actually greater, intensity of the fasted image had to be increased 1000-fold in order to view