VSEPR Shapes
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What is a steric group?
An atom or lone pair that exerts repulsions due to their electrons
What shape does a diatomic molecule form
Linear, two molecules
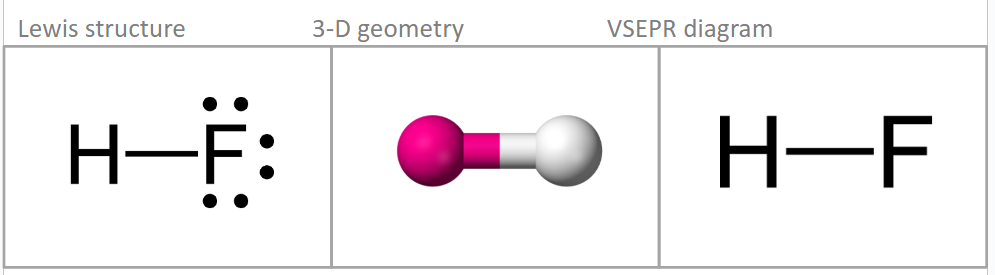
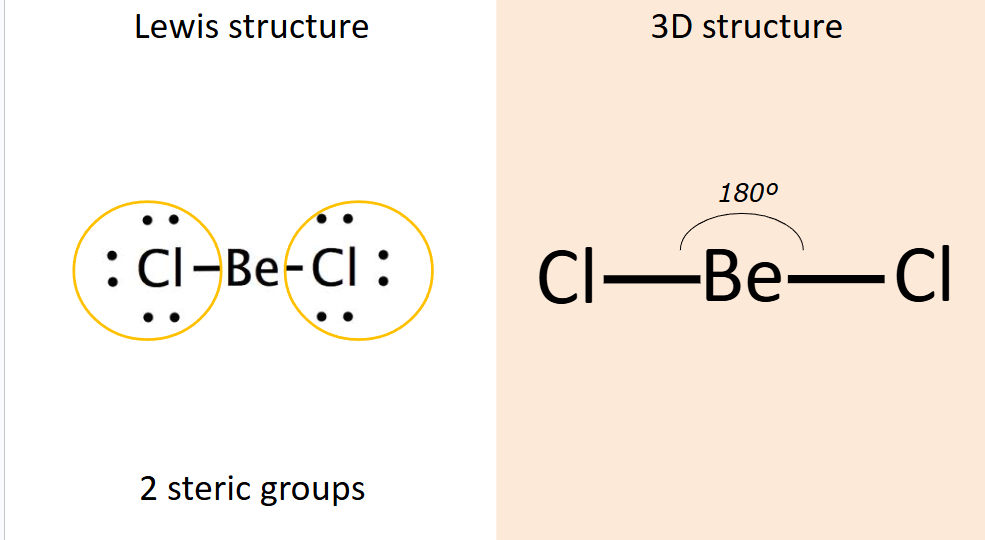
What shape does 2 steric groups form?
Linear
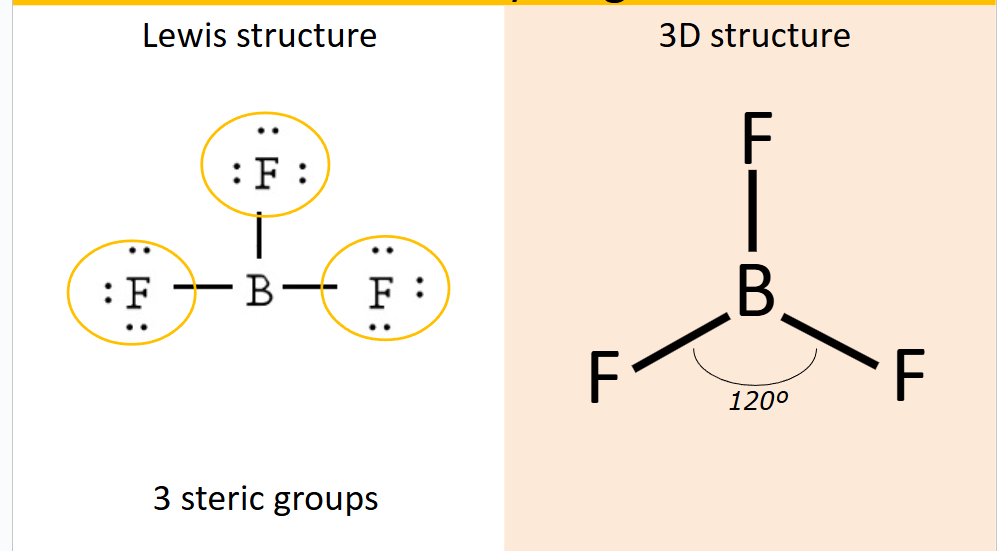
What shape does 3 steric groups form?
Trigonal Planar, 120 degrees between each group
What about the molecular geometry of 3 steric groups, but one of them is a lone pair?
bent, angular
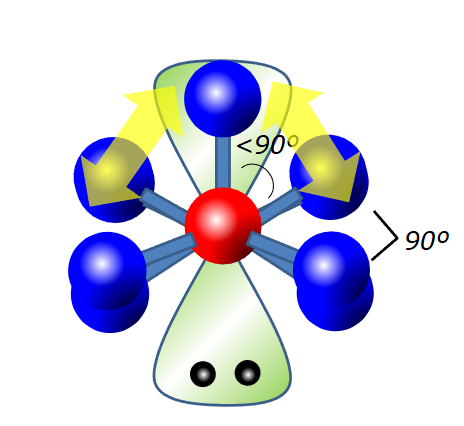
What electron geometry does 4 steric groups make
Tetrahedral, 109.5 degrees
What is the molecular geometry of 4 steric groups but one is a lone pair? What about 2 lone pairs?
Trigonal pyramidal, bent
What is the electron geometry of 5 steric groups
Trigonal bipyramidal
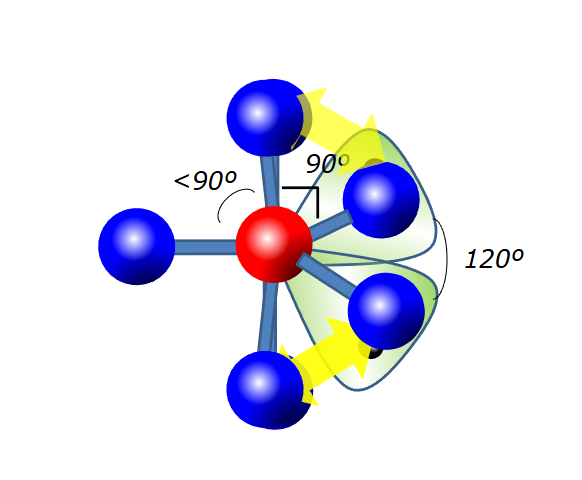
What is the molecular geometry of 5 steric groups but one is a lone pair. What about 2 lone pairs? 3?
Seesaw, t-shape, linear
What is the electron geometry of 6 steric groups
Octahedral, 90 degrees
What is the electron geometry of 6 steric groups, but one lone pair. what bout 2?
square pyramidal, square planar