Biochem Exam 3 Stuff
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
Enzymes
The workers of the cell
Enzymes are proteins that carry out a chemical reaction (Note that RNAs can also do that!)
Enzymes act as catalysts of chemical reactions so the reactions occur at a much faster rate than uncatalyzed reactions
As we will see, they do so through two mechanisms:
By providing a protected environment
By providing all of the reactive partners [acids (Asp, Glu, C-term), bases (Lys, Arg, His, N-term), and hydrogen-bonding] in the correct configuration
General Properties of Enzymes:
Higher reaction rates
106 - 1012 times greater than those of uncatalyzed reactions and at least ~107 times greater than chemically catalyzed reactions
General Properties of Enzymes:
Milder reaction conditions
Near neutral pH, < 100°C, atmospheric pressure
Chemical catalysis often requires high T, pressure, and pH
General Properties of Enzymes:
Greater reaction specificity
Enzymatic reactions are generally highly specific for substrates and therefore rarely produce any side products
General Properties of Enzymes:
Capacity for regulation
Enzymatic catalysis can be regulated
Allosteric control, covalent modification, and variations in synthesis of the protein (i.e. protein expression)
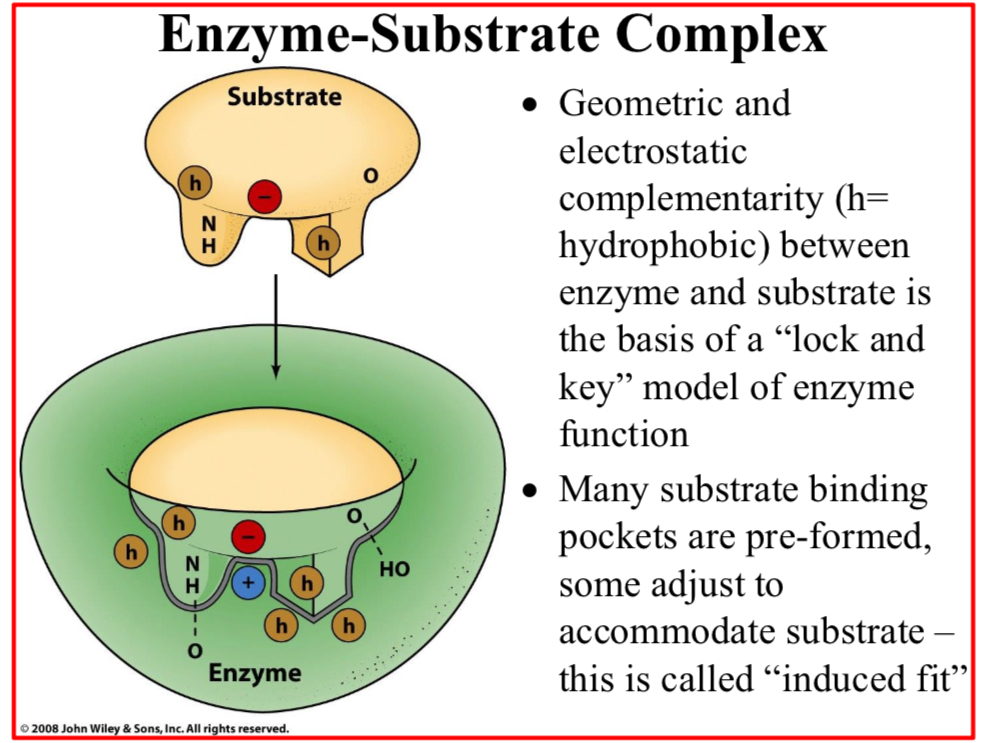
Geometric and electrostatic complementarity (h=hydrophobic) between enzyme and substrate is…
The basis of a “lock and key” model of enzyme function
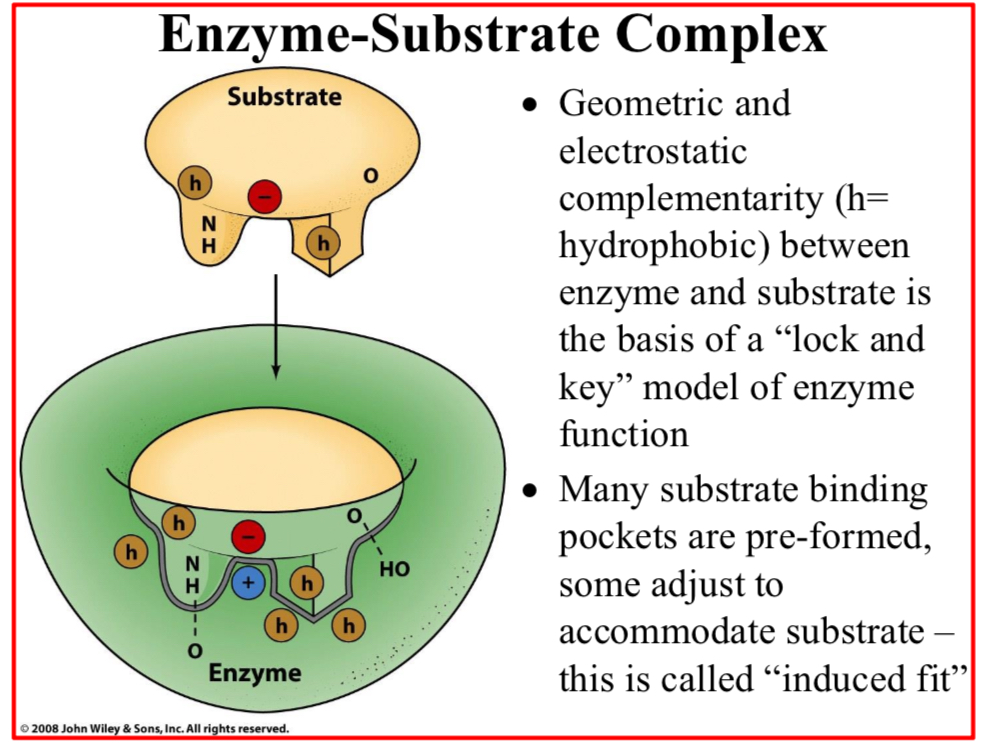
Many substrate binding pockets are pre-formed,…
Some adjust to accommodate substrate – this is called “induced fit”
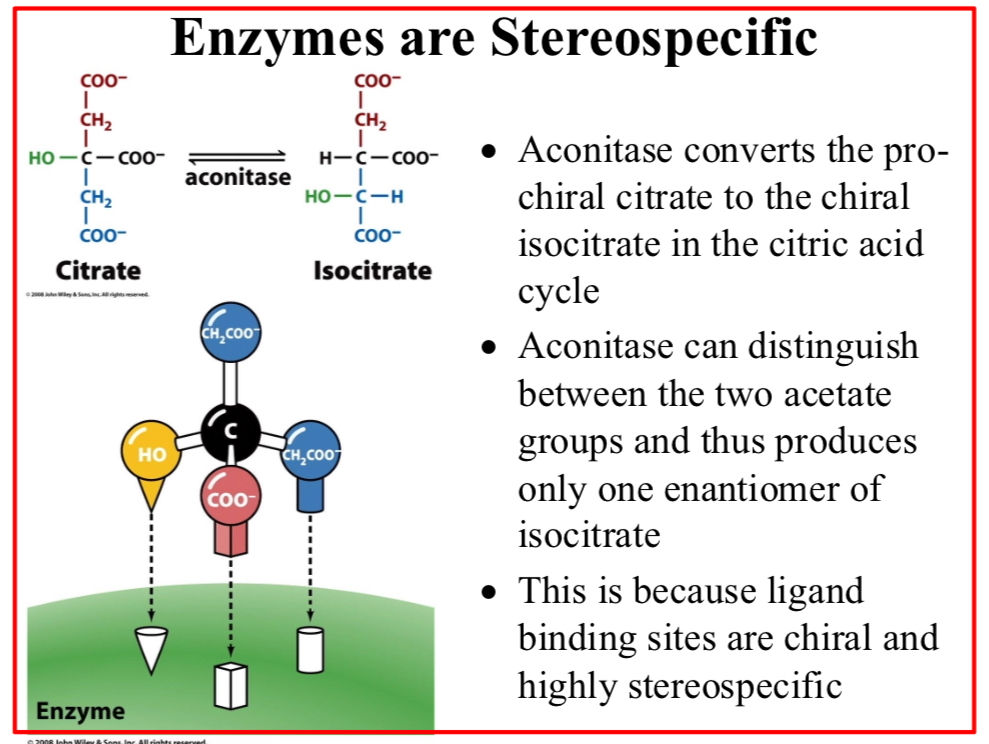
Enzymes are stereospecific
Aconitase converts the pro-chiral citrate to the chiral isocitrate in the citric acid cycle
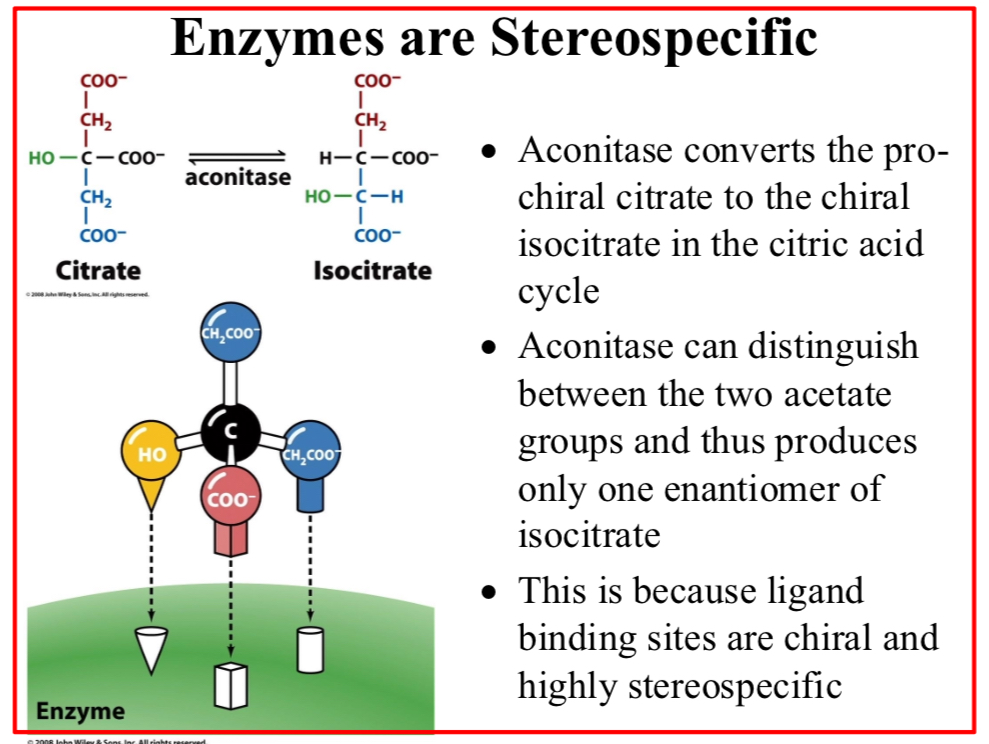
Aconitase can distinguish between the two acetate groups and thus produces only one enantiomer of isocitrate
This is because ligand binding sites are chiral and highly stereospecific

Preferentially or exclusively bind one enantiomer and…
Carries out stereospecific reactions

Recall that enzymes are constructed of chiral amino acids
These chiral amino acids create a chiral substrate binding pocket

Even when the substrate of a reaction is not chiral, catalysis proceeds stereospecifically and…
Often leads to a chiral product

L-proteins act on specific chirality, therefore…
D-proteins will act on opposite chirality
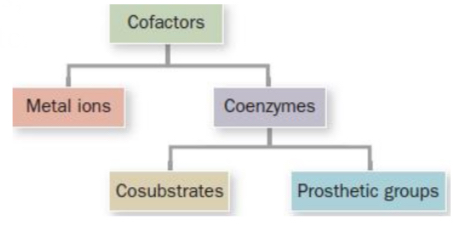
Cofactors
Molecular modules that enable enzymes to expand their repertoire of chemistry!
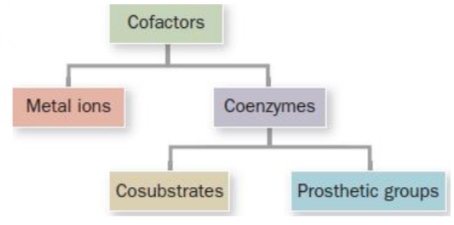
Metal ions
Essential non-protein elements of some enzymes
Cu2+, Fe3+, Zn2+, etc.
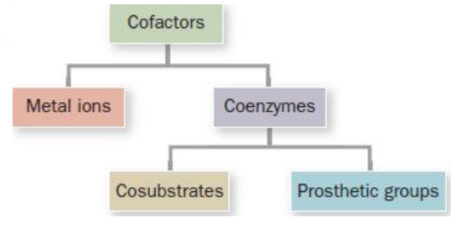
Coenzymes
Organic/biological cofactors
Cosubstrates & Prosthetic groups
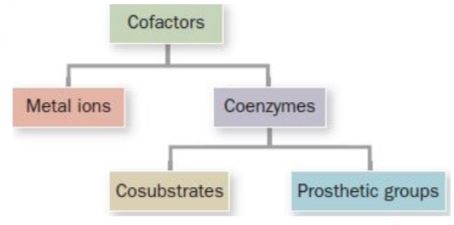
Cosubstrates
Transiently enzyme-associated coenzymes
NAD+, NADP+, etc.
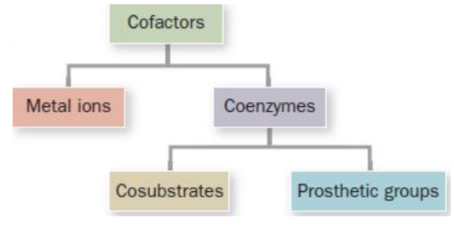
Prosthetic groups
Permanently enzyme-associated coenzymes
Heme, pyridoxal phosphate, etc
Coenzymes are generally reduced/oxidized during a reaction and must be returned to their initial state
This can be done by the same enzyme or a separate one
Holoenzyme
Catalytically active enzyme-cofactor complex
Apoenzyme
Inactive protein resulting from removal of cofactor
Apoenzyme (inactive) + cofactor ↔ …
Holoenzyme (active)
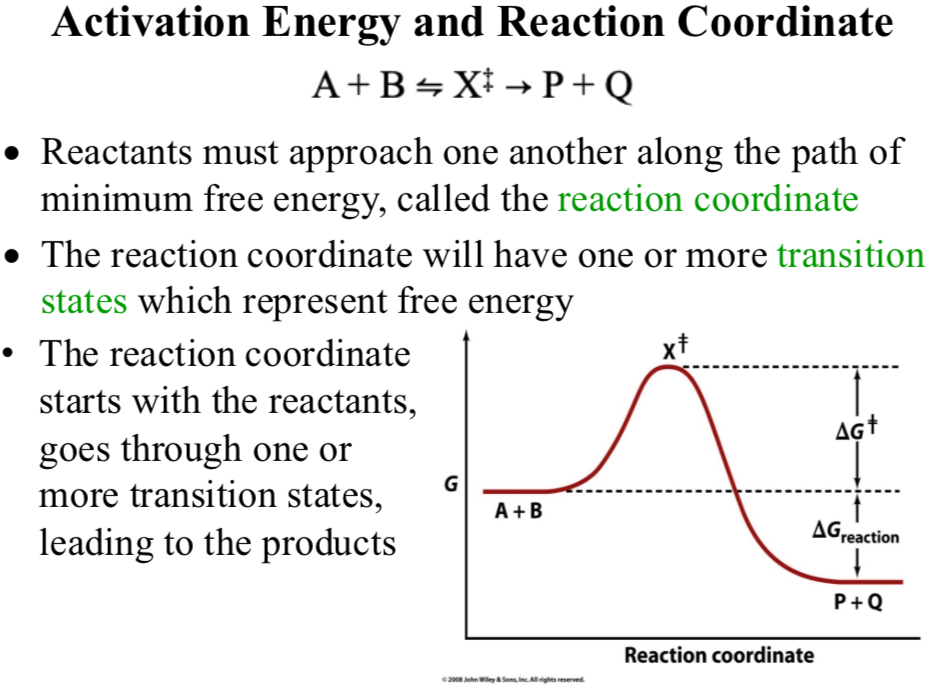
Activation Energy and Reaction Coordinate
A + B ⇌ X‡ → P + Q
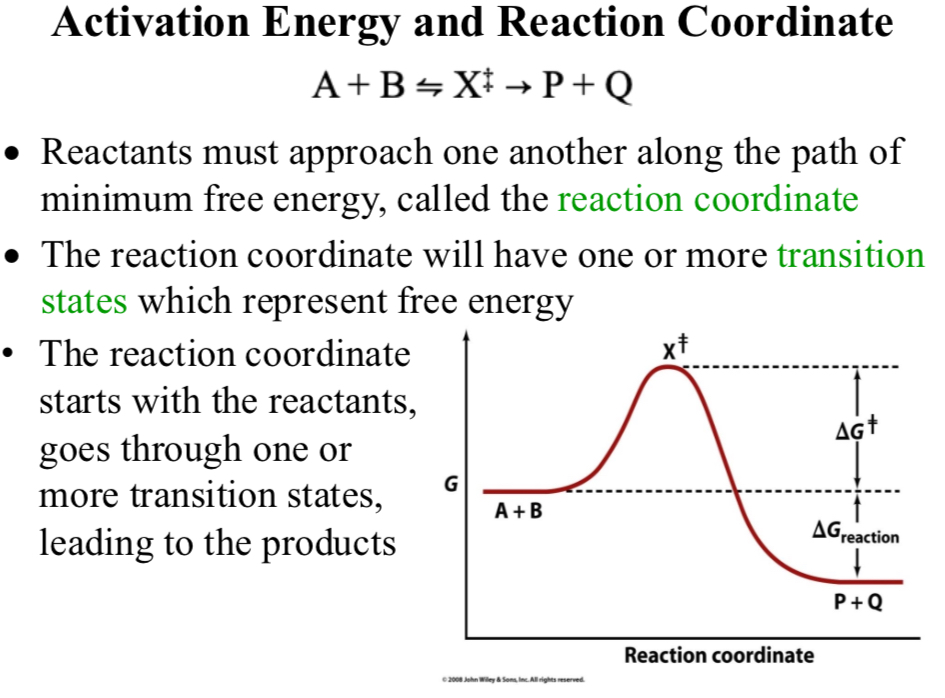
Reaction coordinate
Reactants must approach one another along the path of minimum free energy
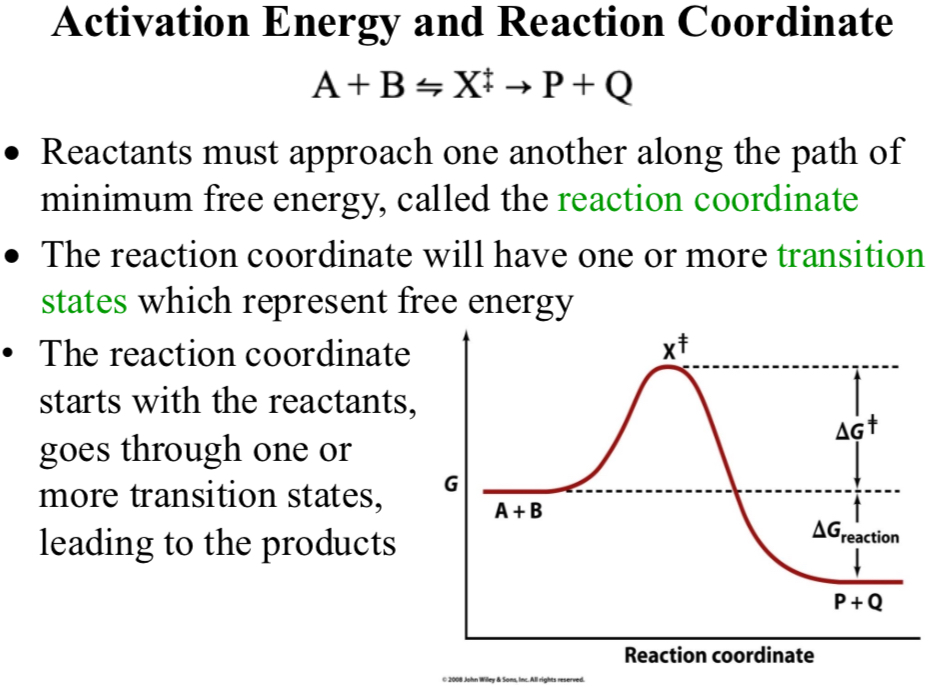
The reaction coordinate will have one or more transition states which represent free energy
The reaction coordinate starts with the reactants, goes through one or more transition states, leading to the product
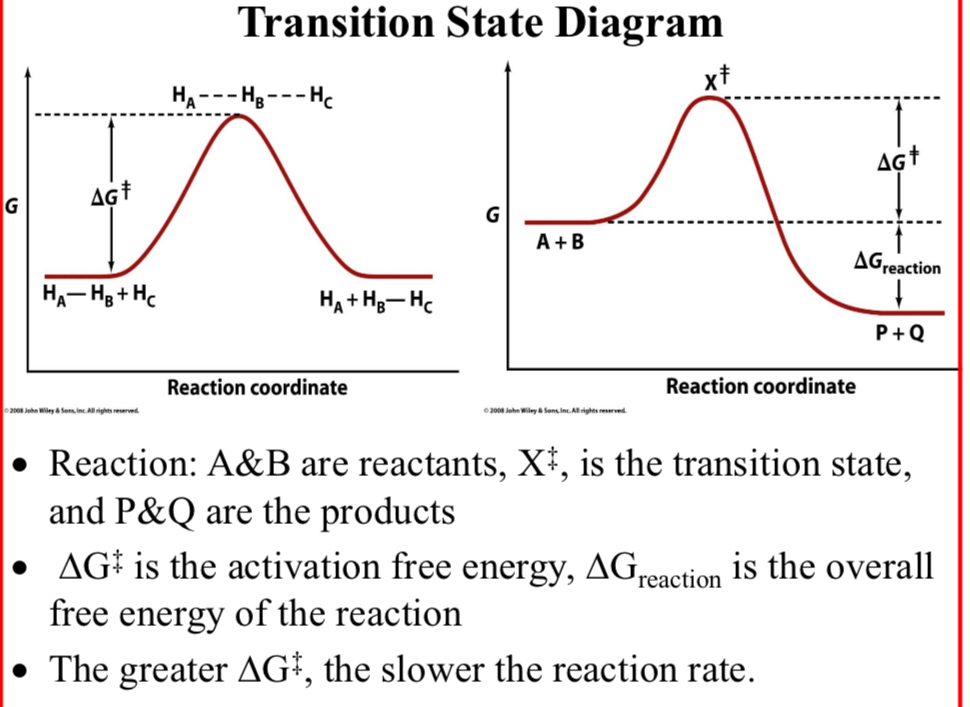
Transition State Diagram; Reaction:
A&B: reactants
X‡: the transition state
P&Q: the products
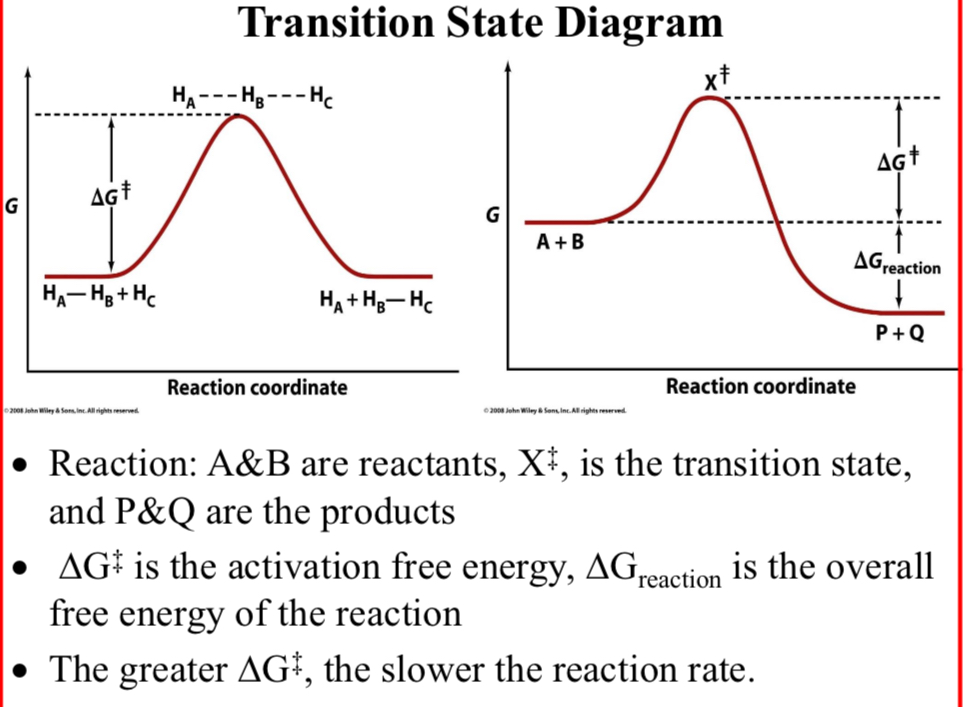
ΔG‡ is the activation free energy
ΔGreaction is the overall free energy of the reaction
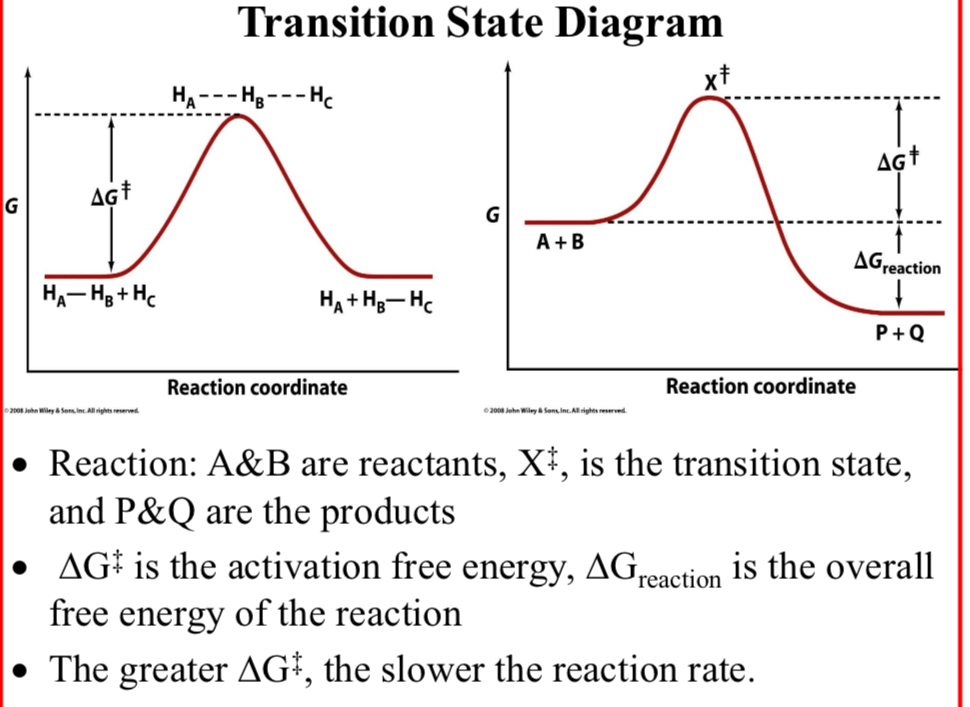
The greater ΔG‡…
The slower the reaction rate
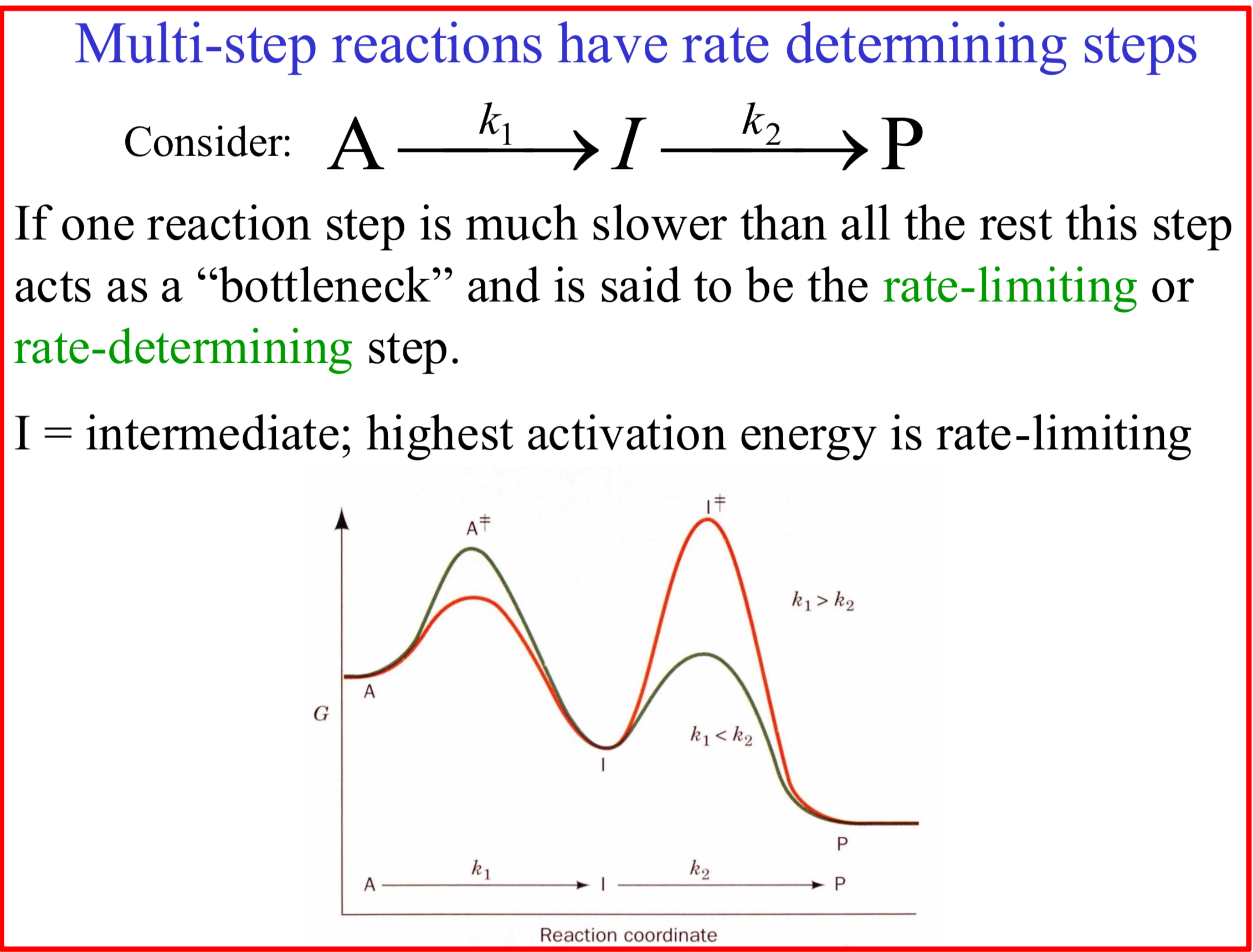
Rate-limiting or rate-determining step
When one reaction step is much slower than all the rest this step acts as a “bottleneck”
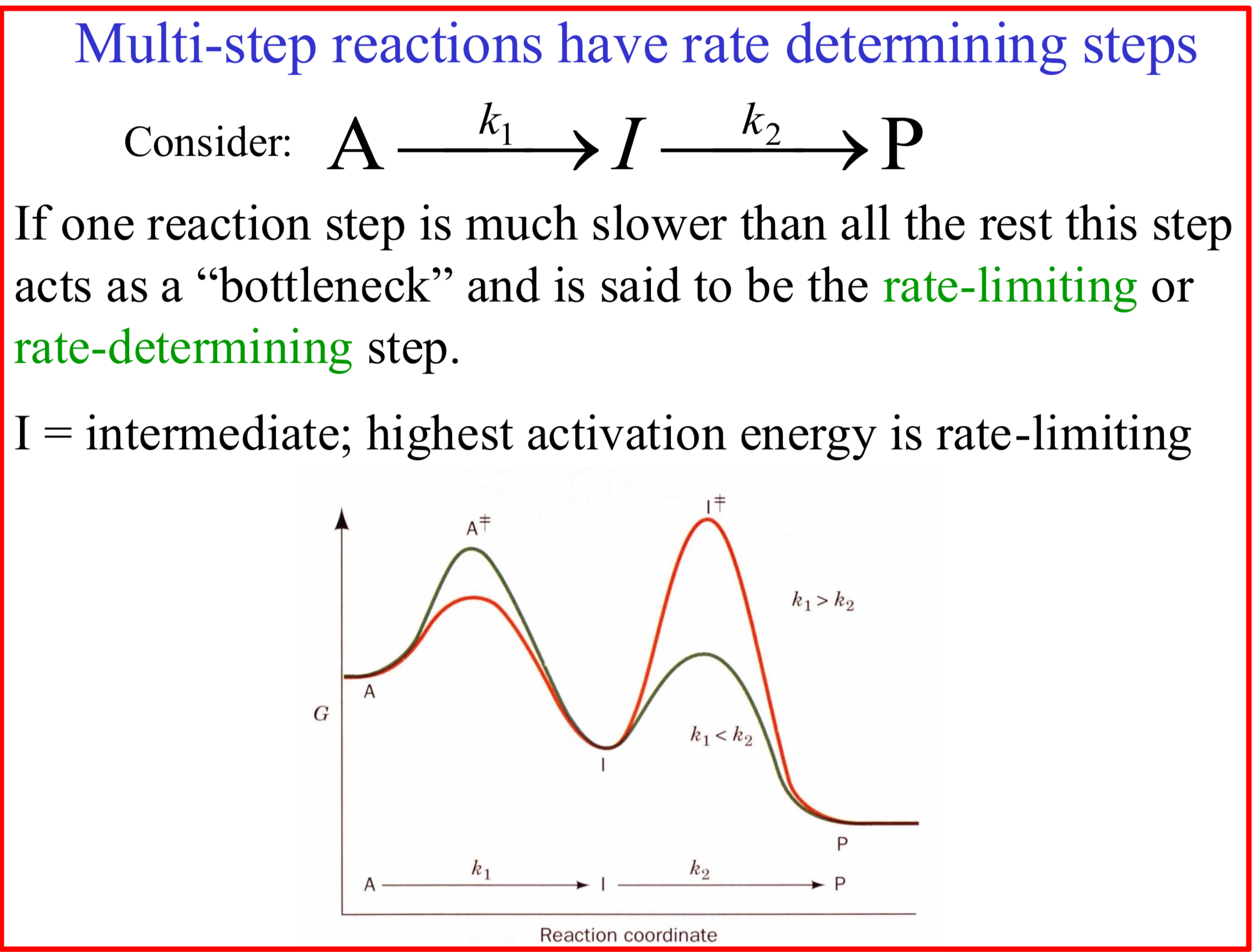
I = intermediate
Highest activation energy is rate-limiting
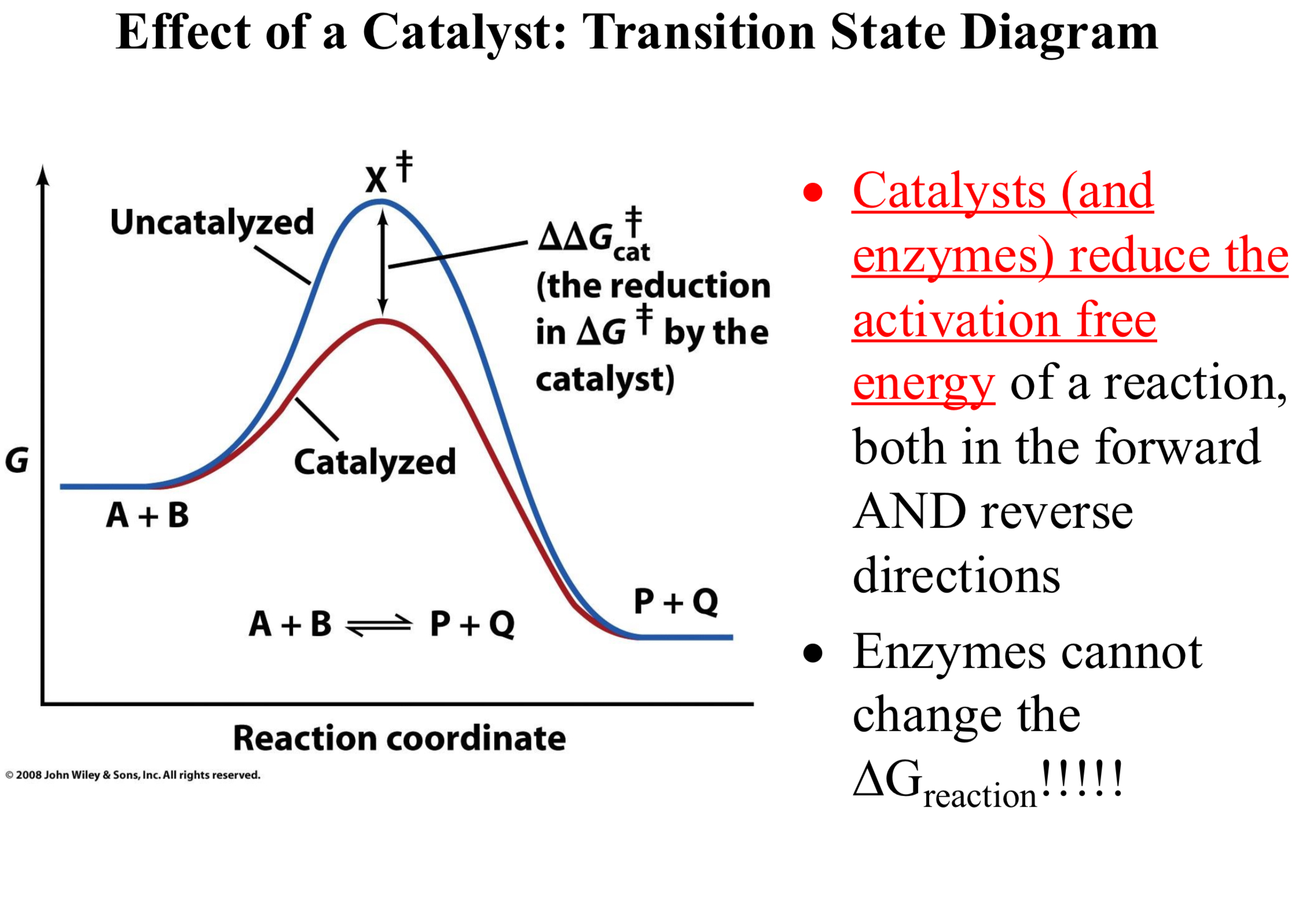
Catalysts (and enzymes) reduce the activation free energy of a reaction, both in the forward AND reverse directions
Enzymes cannot change the ΔGreaction!!!!
Catalytic Mechanisms; Enzymes use multiple different mechanisms to catalyze biochemical reactions:
Acid-base catalysis
Covalent catalysis
Metal ion catalysis
Electrostatic catalysis
Proximity and orientation effects
Preferential binding of the transition state complex
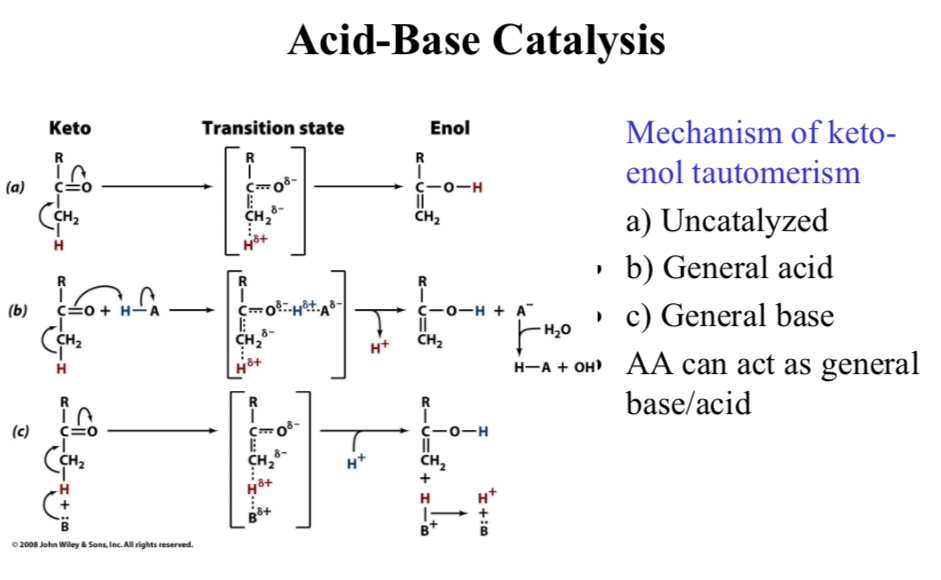
Acid-Base Catalysis
Mechanism of keto-enol tautomerism
a) Uncatalyzed
b) General acid
c) General base
AA can act as general base/acid
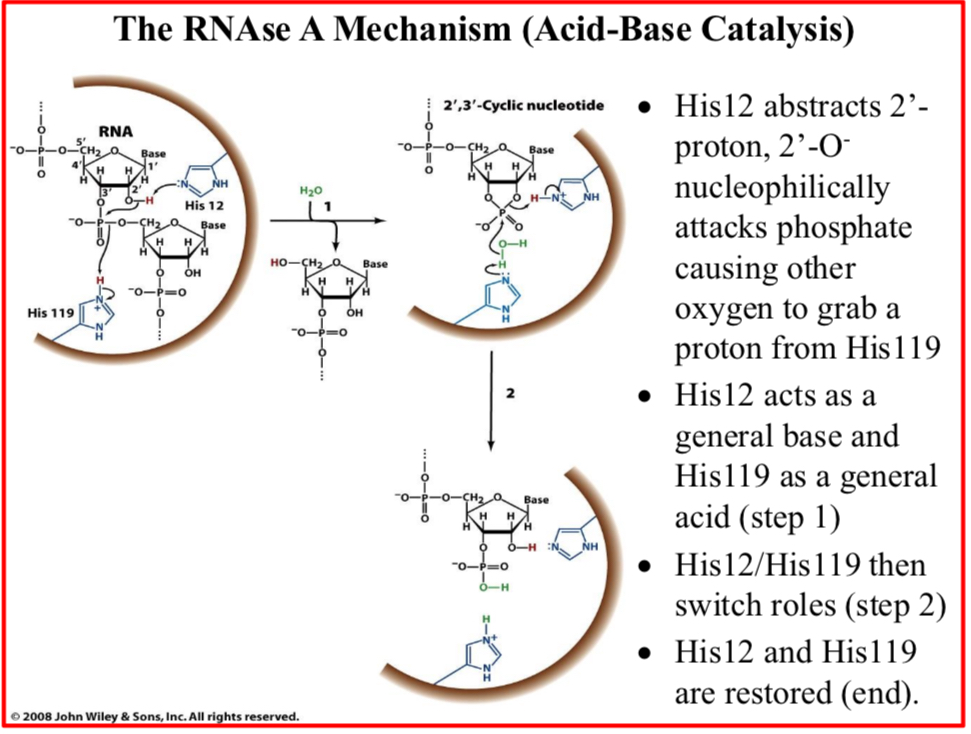
His12 abstracts 2’-proton, 2’-O- nucleophilically attacks phosphate causing other oxygen to grab a proton from His119
His12 acts as a general base and His119 as a general acid (step 1)
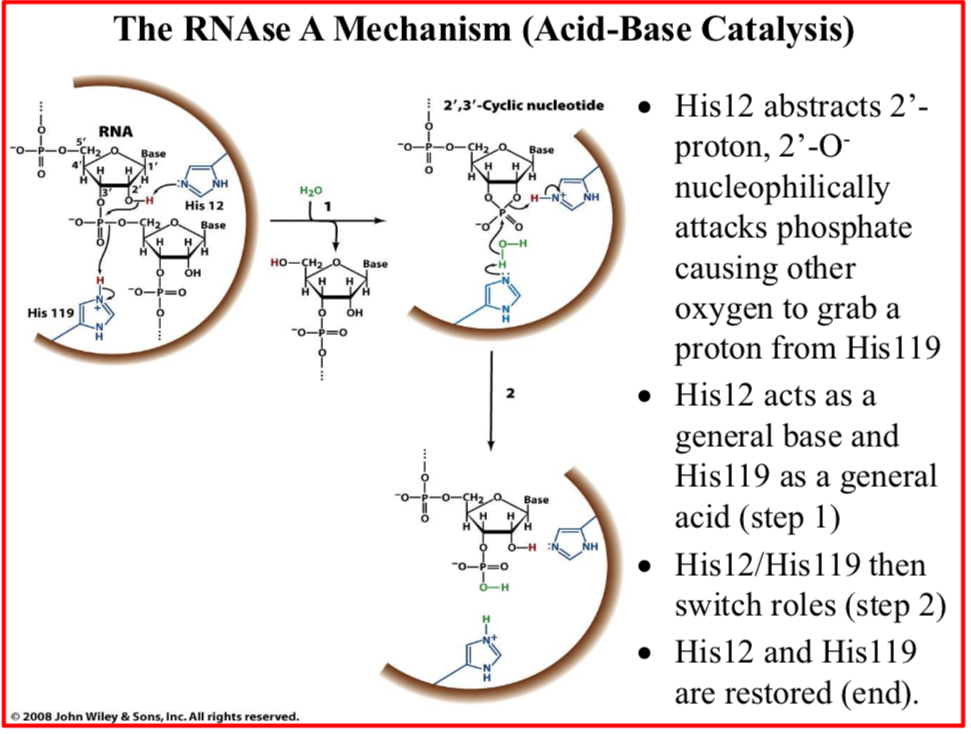
His12/His119 then switch roles (step 2)
His12 and His119 are restored (end)
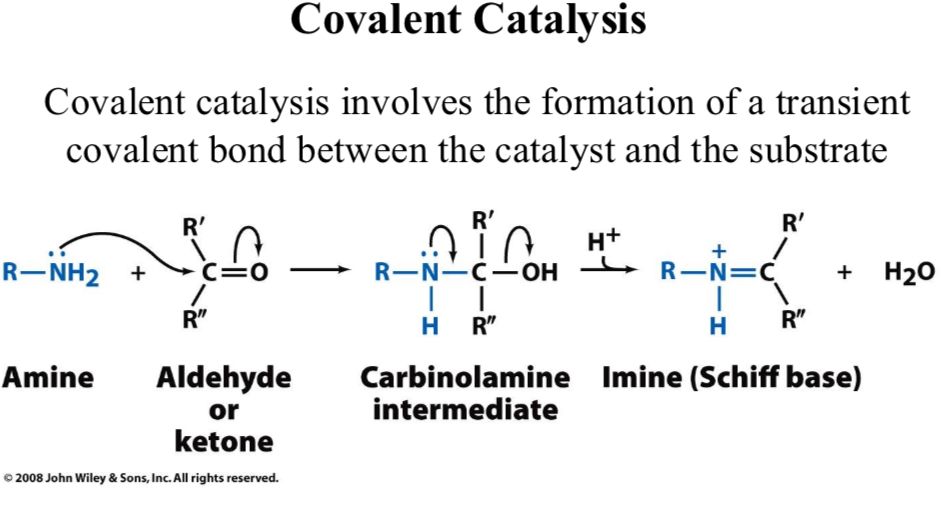
Covalent Catalysis
Covalent catalysis involves the formation of a transient covalent bond between the catalyst and the substrate
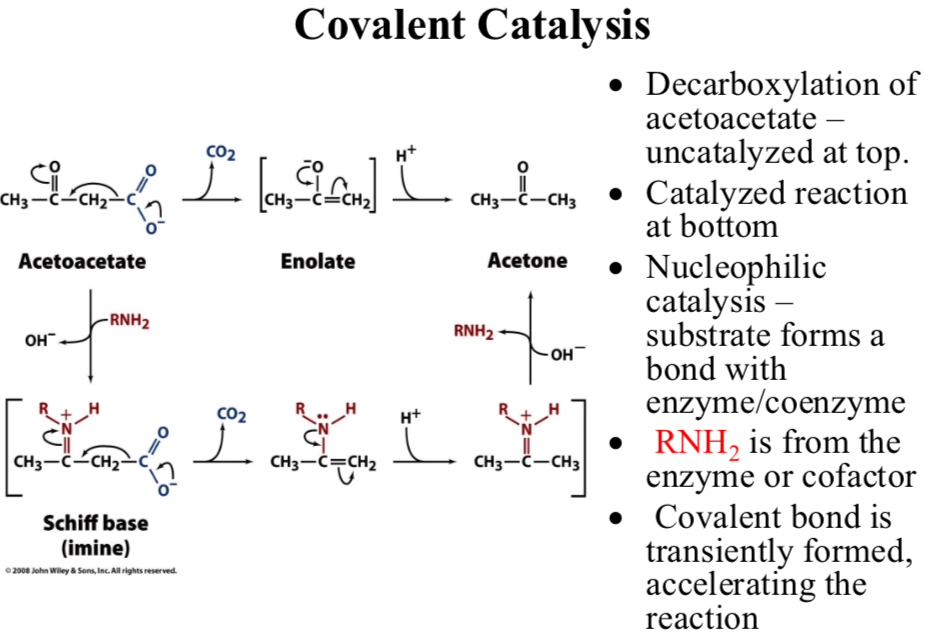
Decarboxylation of acetoacetate:
Uncatalyzed at top
Catalyzed reaction at bottom
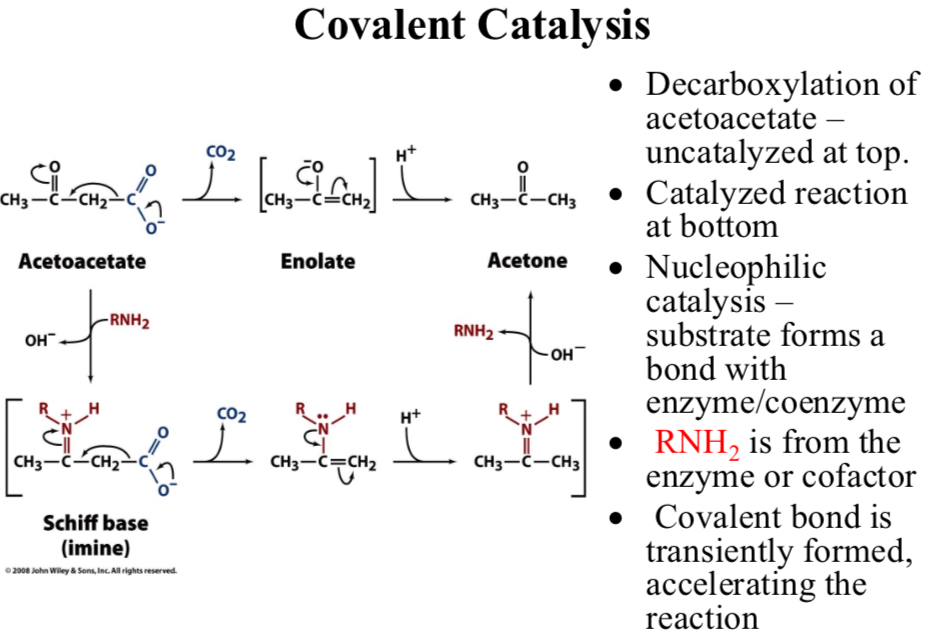
Nucleophilic catalysis:
Substrate forms a bond with enzyme/coenzyme
RNH2 is from the enzyme or cofactor
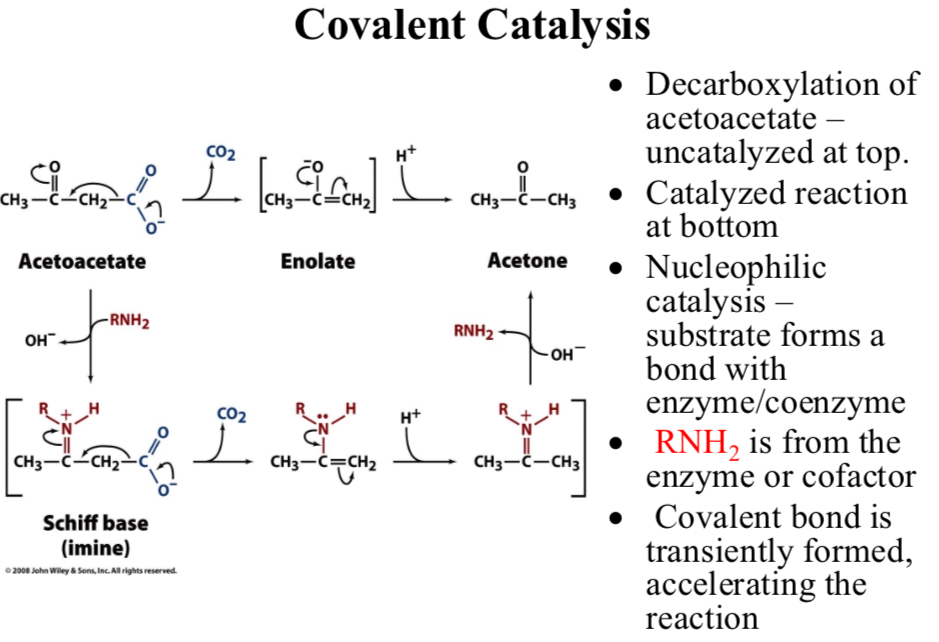
Covalent bond is transiently formed, …
Accelerating the reaction
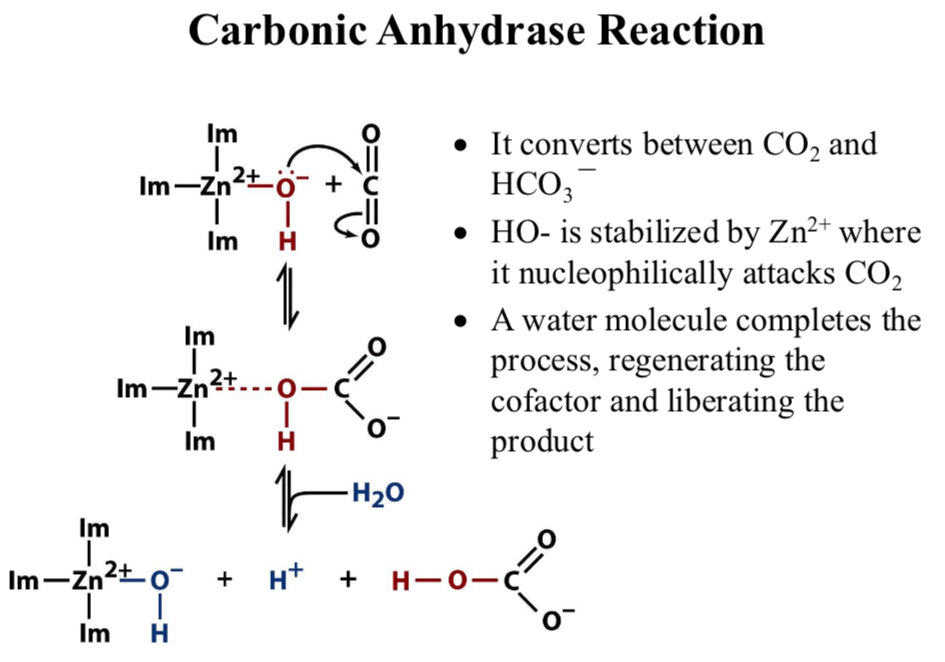
Carbonic Anhydrase Reaction
It converts between CO2 and HCO3-
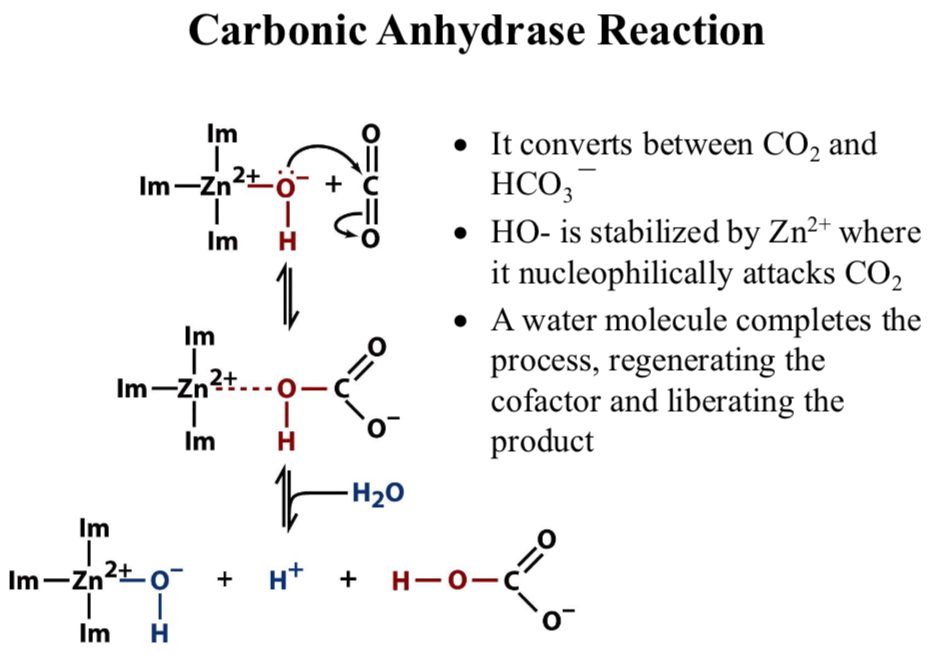
HO- is stabilized by Zn2+ where it nucleophilically attacks CO2
A water molecule completes the process, regenerating the cofactor and liberating the product
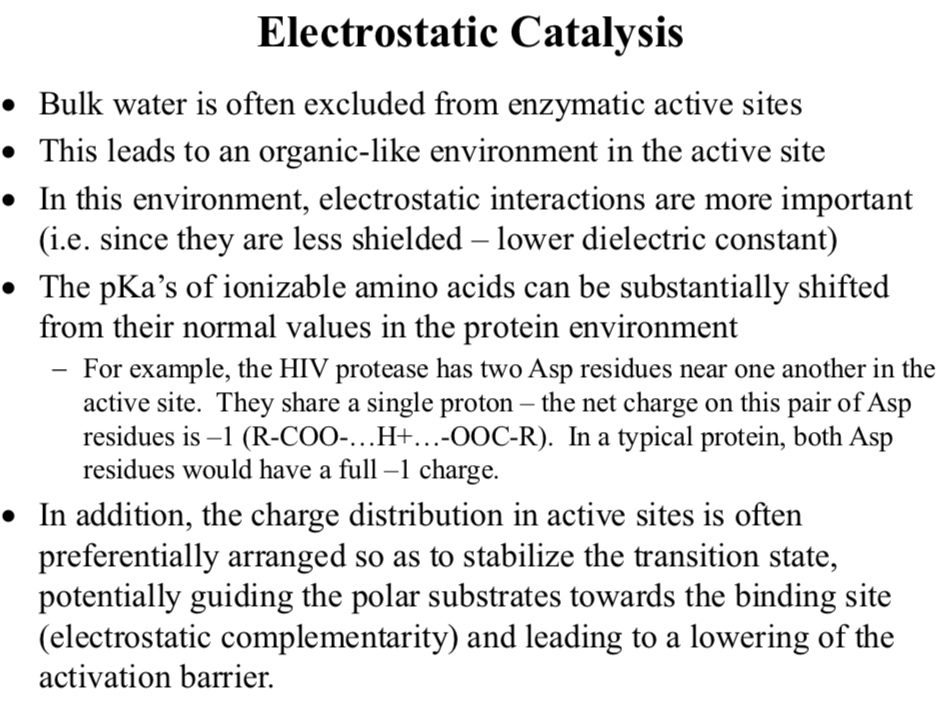
Electrostatic Catalysis
Bulk water is often excluded from enzymatic active sites
This leads to an organic-like environment in the active site
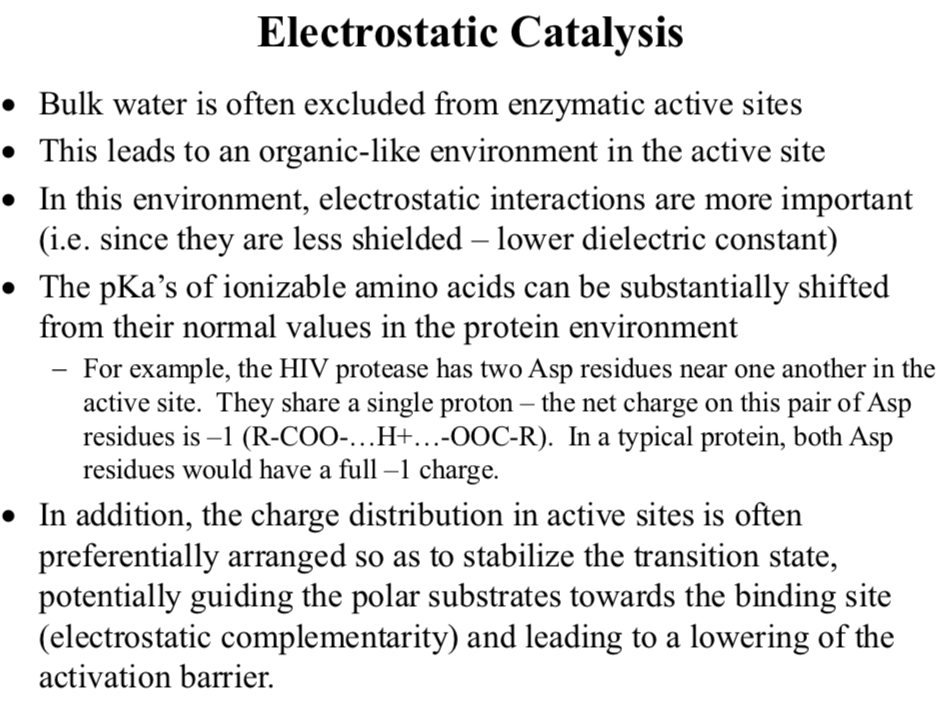
In this environment, electrostatic interactions are more important
i.e. since they are less shielded – lower dielectric constant
The pKa’s of ionizable amino acids can be substantially shifted from their normal values in the protein environment
For example, the HIV protease has two Asp residues near one another in the active site. They share a single proton – the net charge on this pair of Asp residues is –1 (R-COO-…H+…-OOC-R). In a typical protein, both Asp residues would have a full –1 charge.
In addition, the charge distribution in active sites is often preferentially arranged so as to stabilize the transition state, potentially guiding the polar substrates towards…
The binding site (electrostatic complementarity) and leading to a lowering of the activation barrier
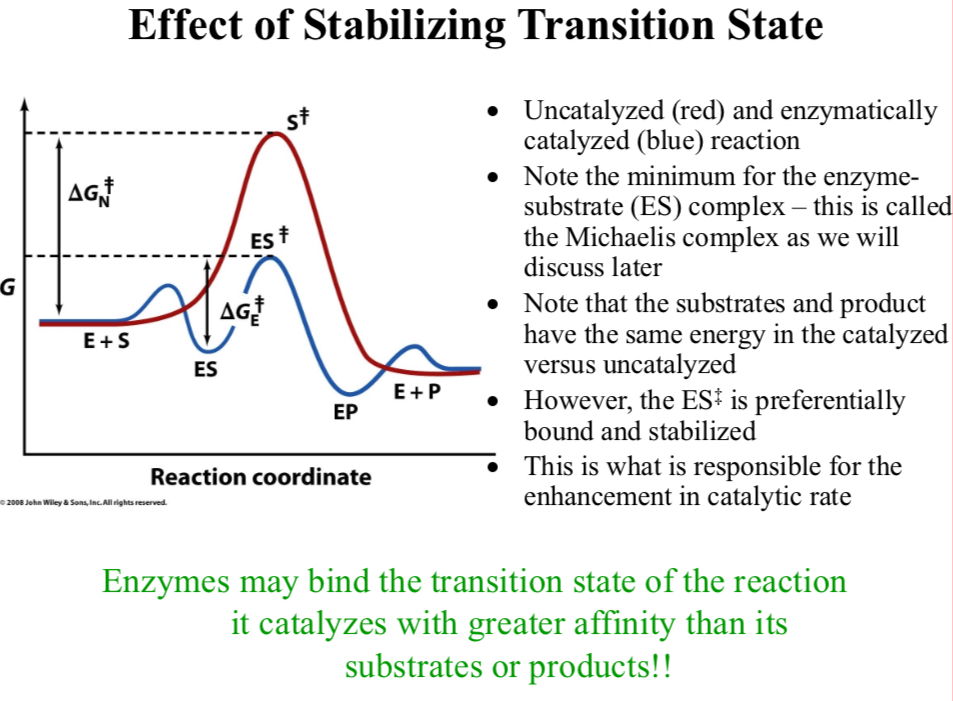
Uncatalyzed (red) and enzymatically catalyzed (blue) reaction
Note the minimum for the enzyme-substrate (ES) complex
It’s called the Michaelis complex
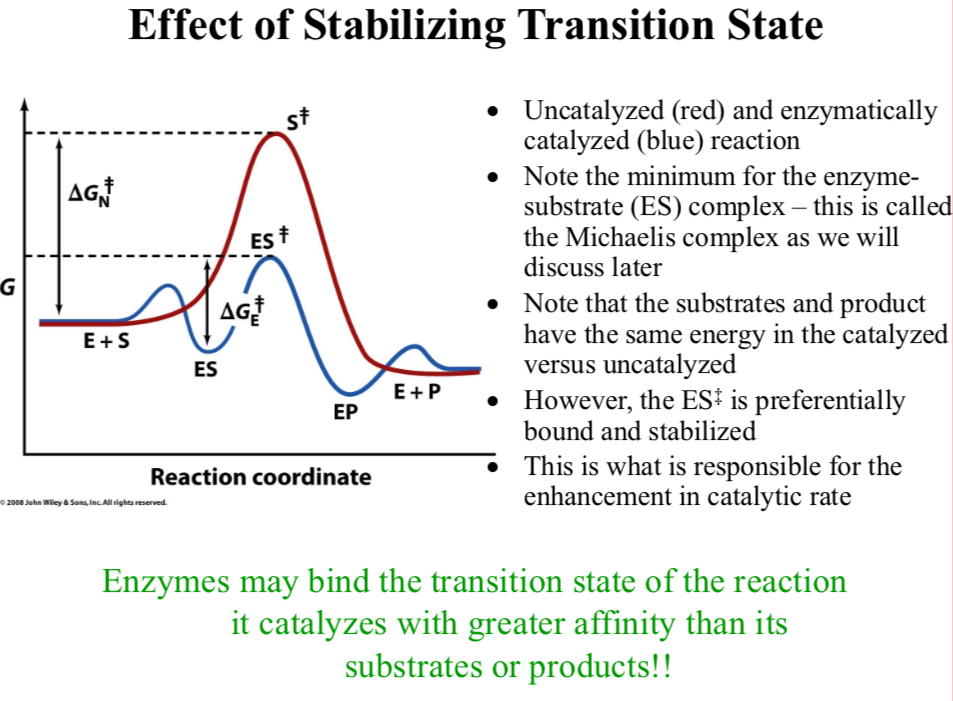
Note that the substrates and product have the same energy in the catalyzed versus uncatalyzed
However, the ES‡ is preferentially bound and stabilized
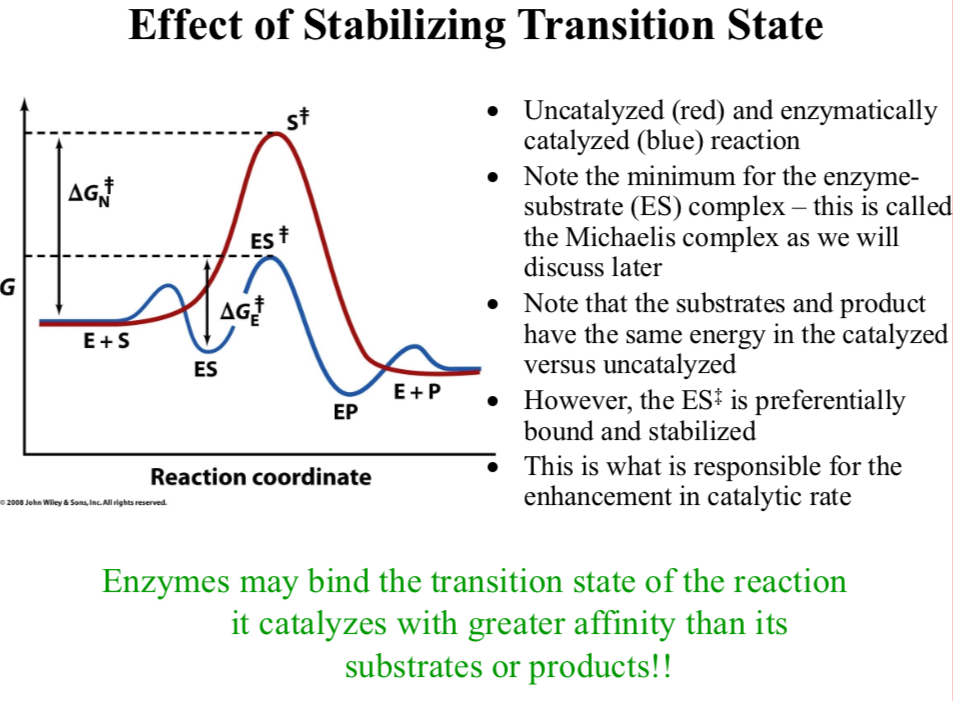
This is what is responsible for the enhancement in catalytic rate
Enzymes may bind the transition state of the reaction it catalyzes with greater affinity than its substrates or products!
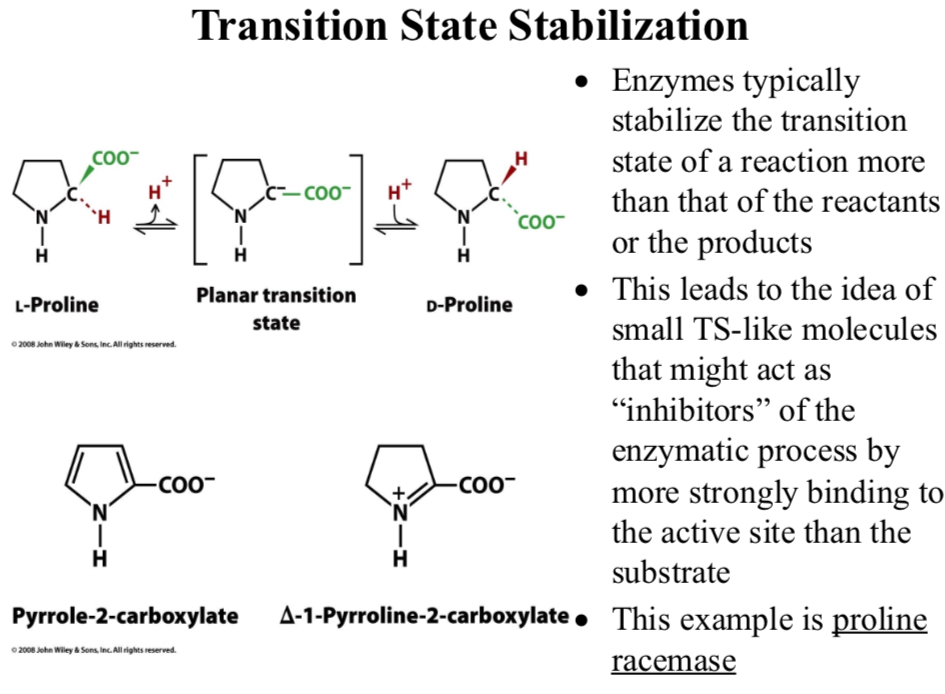
Enzymes typically stabilize the transition state of a reaction more than that of the reactants or the products
This leads to the idea of small TS-like molecules that might act as “inhibitors” of the enzymatic process by more strongly binding to the active site than the substrate