chemistry ch 11
5.0(1)
5.0(1)
Card Sorting
1/64
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
1
New cards
kinetic theory
all matter is comprised of small constantly moving particles experiencing collisions in which energy is conserved (perfectly elastic)
2
New cards
compressibility
property of matter arising from the empty spaces among particles
3
New cards
fluidity
property of matter arising from the ease of movement of particles relative to each other
4
New cards
incompressible
not fluid
not fluid
what can be said about the compressibility and fluidity of solids?
5
New cards
low compressibility
fluid
fluid
what can be said about the compressibility and fluidity of liquids?
6
New cards
high compressibility
fluid
fluid
what can be said about the compressibility and fluidity of gases?
7
New cards
ideal gas
PV=nRT
no attractive forces
point masses (no volume)
no attractive forces
point masses (no volume)
8
New cards
real gas
(P = n^2a/v)(V-nb)=nRT
a -> dependent on magnitude of attractive forces
b -> dependent on size of molecules
a -> dependent on magnitude of attractive forces
b -> dependent on size of molecules
9
New cards
liquids and solids
no simple equation bc you can't neglect/fix two things:
1) IMFs
2) particle size (relative to volume)
1) IMFs
2) particle size (relative to volume)
10
New cards
sublimation
solid to gas
11
New cards
deposition
gas to solid
12
New cards
condensation
gas to liquid
13
New cards
vaporization/evaporation
liquid to gas
14
New cards
melting/fusion
solid to liquid
15
New cards
freezing/crystallization
liquid to solid
16
New cards
vapor pressure
the equilibrium partial pressure of the vapor over the liquid (or solid) at a given temperature (ex: Pwater)
17
New cards
maxwell-boltzman distribution graph
graph that shows us liquid to gas phases, where the area under the curve is the # of particles able to escape into the gas phase
18
New cards
boiling point
temperature at which the Pvap of the liquid is equal to the pressure on the liquid surface (Patm unless the vessel is closed)
19
New cards
normal boiling point
T at which Pvap = 1 atm = 760 mmHg
20
New cards
freezing point
temperature at which the pure liquid changes to a crystalline solid
21
New cards
melting point
temperature at which the pure crystalline solid changes to a liquid
22
New cards
pressure changes
freezing and melting point are both virtually unaffected by ...
23
New cards
molecular
which type of substance is the easiest to melt/boil
24
New cards
covalent network
which type of substance is the hardest to melt/boil
25
New cards
temperature change to see phase changes
what does this graph show us
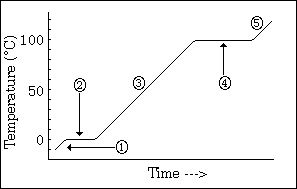
26
New cards
-phase changes
-all energy goes into changing phases not changing temperature (when it hits equilibrium
-all energy goes into changing phases not changing temperature (when it hits equilibrium
what do the horizontal lines indicate, and why does the temperature remain constant
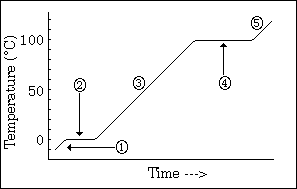
27
New cards
long line > high IMF; high energy required to change phase > long line
what does the length of each line indicate
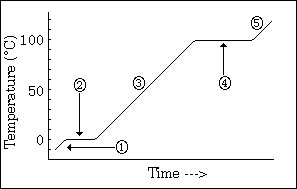
28
New cards
sloped line represents 1 phase
high steepness = low specific heat
high steepness = low specific heat
what does a sloped line represent?
and what does the steepness indicate about specific heat?
and what does the steepness indicate about specific heat?
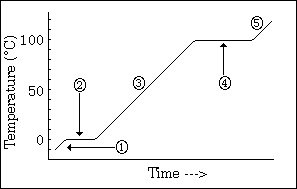
29
New cards
clausius-clapeyron equation
related Pvap to Tk of its liquid
NOTE: slope: negative; use R= 8.31 J/mol K
need to find B value for each substance
ln P = (-change in Hvap/R) (1/T) + B
NOTE: slope: negative; use R= 8.31 J/mol K
need to find B value for each substance
ln P = (-change in Hvap/R) (1/T) + B
30
New cards
two point form
use the log form at two T's, T1 and T2 and subtract equations
ln (P2/P1) = change in Hvap/R (1/T1 - 1/T2)
ln (P2/P1) = change in Hvap/R (1/T1 - 1/T2)
31
New cards
1 atm or 760 mmHg
if a problem says normal boiling point in it that means the pressure = ...
32
New cards
phase diagram
a graphic to summarize the conditions, T and P, under which different states of a substance are stable
33
New cards
area regions
condition where a given phase is stable
34
New cards
line segments
conditions where two phases exist in equilibrium
35
New cards
specific heat
the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree
36
New cards
triple point (TP)
conditions where all three phases exist in equilibrium
37
New cards
critical point (CP)
T above which the liquid does not exist regardless of the pressure (Ek has totally overcome all attractive forces)
38
New cards
surface tension
energy required to increase the surface area of liquid by a unit amount; results from the net force toward the interior of the liquid experienced by a surface molecule
39
New cards
capillary rise
phenomenon related to the surface tension
-water molecules attract to glass, bc of this attraction, a thin film of water starts to move up inside of the glass capillary, thus making a meniscus
-water molecules attract to glass, bc of this attraction, a thin film of water starts to move up inside of the glass capillary, thus making a meniscus
40
New cards
viscosity
the resistance to flow that is exhibited by all liquids and gases
41
New cards
liquid is slow
high viscosity, really viscous
42
New cards
easy to pour
low viscosity
43
New cards
imfs
the forces of interaction between molecules
(typically weakly attractive; abt 100 to 1000 times smaller than bond energies)
(typically weakly attractive; abt 100 to 1000 times smaller than bond energies)
44
New cards
van der waals forces
weak attractive forces (LDF and dipole-dipole)
45
New cards
london dispersion forces
weak intermolecular attractive forces resulting from the small, instantaneous, synchronized dipoles occurring due to the varying positions of the electrons of the electrons as they move about the nuclei
46
New cards
increase w molar mass
ldfs tend to
47
New cards
polarizable
larger atoms are more
48
New cards
polarizable
molecules that are more spread out tend to be more
49
New cards
dipole dipole forces
one polar molecule's partial positive charge is attracted to the partial negative of another polar molecule
50
New cards
stronger
LDF is __ than DD but DD is permanent
51
New cards
weak Pvap
strong surface tension
high normal boiling points
high viscosity
strong surface tension
high normal boiling points
high viscosity
strong IMFS yield
52
New cards
ion dipole
the attractive force between a polar molecule and a charge particle ion
-the attraction must be between a partial negative and cation or partial positive and anion
-the attraction must be between a partial negative and cation or partial positive and anion
53
New cards
hydrogen bonding
a weak to moderate attractive force that exists between an H atom covalently bonded to an N, O, or F atom and an N, O, or F atom on another molecule
54
New cards
hydrogen bonding
___ receives its strength from the partial exposure of the proton of the H nucleus when it is bonded to a highly electronegative atom
55
New cards
molecular
consists of atoms or molecules held together by IMFS; NM/NM
56
New cards
metallic
consists of positive cores of atoms held together by a "sea" of mobile electrons; M
57
New cards
ionic
consists of cations and anions held together by the electrical attraction of opposite charges; M/NM
58
New cards
covalent network
consists of atoms held together in large 3D or 2D networks or chains
59
New cards
C (diamond)
C (graphite)
C60
SiO2
SiC
C (graphite)
C60
SiO2
SiC
five covalent networks
60
New cards
LDF, DD/HB, Ionic/Metallic, Covalent Network
list all the force and bond types in order from weakest to strongest
61
New cards
-high melting points
-brittle, hard
-poor conductors of heat and electricity
-dull
-brittle, hard
-poor conductors of heat and electricity
-dull
ionic solid characteristics
62
New cards
-low melting points
-nonconductors
-soft
-dull
-nonconductors
-soft
-dull
molecular solid characteristics
63
New cards
-HIGHEST melting points
-hard
-dull
-nonconductors
-hard
-dull
-nonconductors
covalent solid characteristics
64
New cards
-variable hardness and melting points (depends on strength of metallic bonding)
-conductors
-lustrous
-ductile
-malleable
-conductors
-lustrous
-ductile
-malleable
metallic solid characteristics
65
New cards
molecular < ionic ~~ metallic < covalent
the general order of increasing strength of interactions in a solid is