6.2.1 Amines
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
What are amines?
Amines are organic compounds derived from ammonia, where one or more hydrogen has been replaced by a carbon chain or ring
What should be noted when drawing the structure of an amine?
The lone pair on the N should be shown
What is an aliphatic amine?
The nitrogen atom is attached to at least one straight or branched carbon chain
Simplest one is methylamine (CH3NH3)
What is an aromatic amine?
The nitrogen atom is attached to an aromatic ring
Simplest is phenylamine (C6H5NH2)
How do we name primary amines?
Adding the suffix -amine to the name of the alkyl chain
How do we name secondary or tertiary amines?
Where a primary amine contains an amine group on any carbon except carbon-1, the amine is named by using amino- and a number to indicate its position
Di- or tri- are used to indicate number of alkyl groups attached
What is an N-substituted derivative?
When two or more different groups are attached to a nitrogen atom, the compound is named as a N-substituted derivative of the larger group
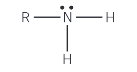
Show the structure of a primary amine
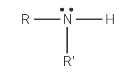
Show the structure of a secondary amine
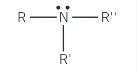
Show the structure of a tertiary amine
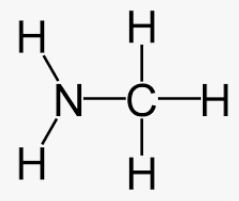
Name this molecule
Methylamine
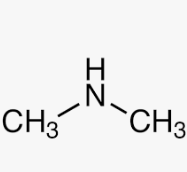
Name this molecule
Dimethylamine
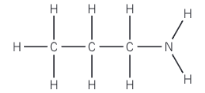
Name this molecule
Propylamine
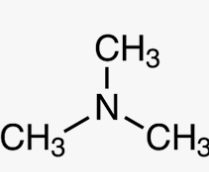
Name this molecule
Trimethylamine
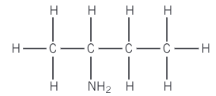
Name this molecule
2-aminobutane
Name this molecule:
CH3NHCH3CH2CH3
N-methylpropylamine
Name this molecule:
CH3N(CH2CH3)CH2CH2CH3
N-ethyl-N-methylpropylamine
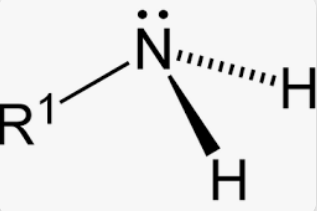
What is the geometric shape and bond angle of an amine?
Pyramidal shape due to extra repulsion of the lone pair
107° bond angle
What is the boiling point of amines like and why?
Amines can form hydrogen bonds so have high boiling points
What is the solubility of amines like and why?
Amines are soluble if short-chained
As the carbon chain length increases, the molecule becomes more non-polar
How do amines behave like bases?
The lone pair of electrons on the nitrogen atom can accept a proton to form a dative covalent bond between the electron lone pair and the proton
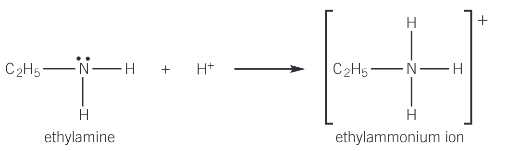
Show how ethylamine acts as a base to form an ion
Which factor affects the base strength of amines? Fill in the blanks:
The ____ of the ____ ____
Alkyl groups have a _____ ______ effect so they push ______ toward the ____
The ‘push’ increases with the _____ of the group, which increases the ______ ______ of the nitrogen, making it more ‘______’
This increases the ability of the _____ _____ on the nitrogen to attract a _______
The size of the alkyl group
Alkyl groups have a positive inductive effect so they push electrons toward the amine
The ‘push’ increases with the size of the group, which increases the charge density of the nitrogen, making it more ‘negative’
This increases the ability of the lone pair on the nitrogen to attract a proton
List in decreasing base strength:
Primary amine
Secondary amine
Tertiary amine
Tertiary
Secondary
Primary
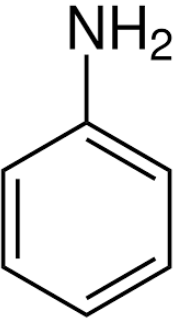
Why is phenylamine a weaker base than ammonia?
Resonance:
The lone pair on the amine is drawn into the delocalised ring
This reduces the ability for the lone pair to attract a proton
Show the reaction behind ammonia and HCl
NH3 + HCl → NH4+Cl-
Give the reaction behind ethylamine and HCl
ethylamine + HCl → ethylammonium chloride
CH3CH2NH2 + HCl → CH3CH2NH3+Cl-
Show the reaction behind ethylamine and sulfuric acid
ethylamine + sulfuric acid → ethylammonium sulfate
2CH3CH2NH2 + H2SO4 → (CH3CH2NH3+)2SO42-
Give the reaction between phenylamine and nitric acid
Phenylamine + nitric acid → phenylammonium nitrate
C6H5NH2 + HNO3 →C6H5NH3+NO3-
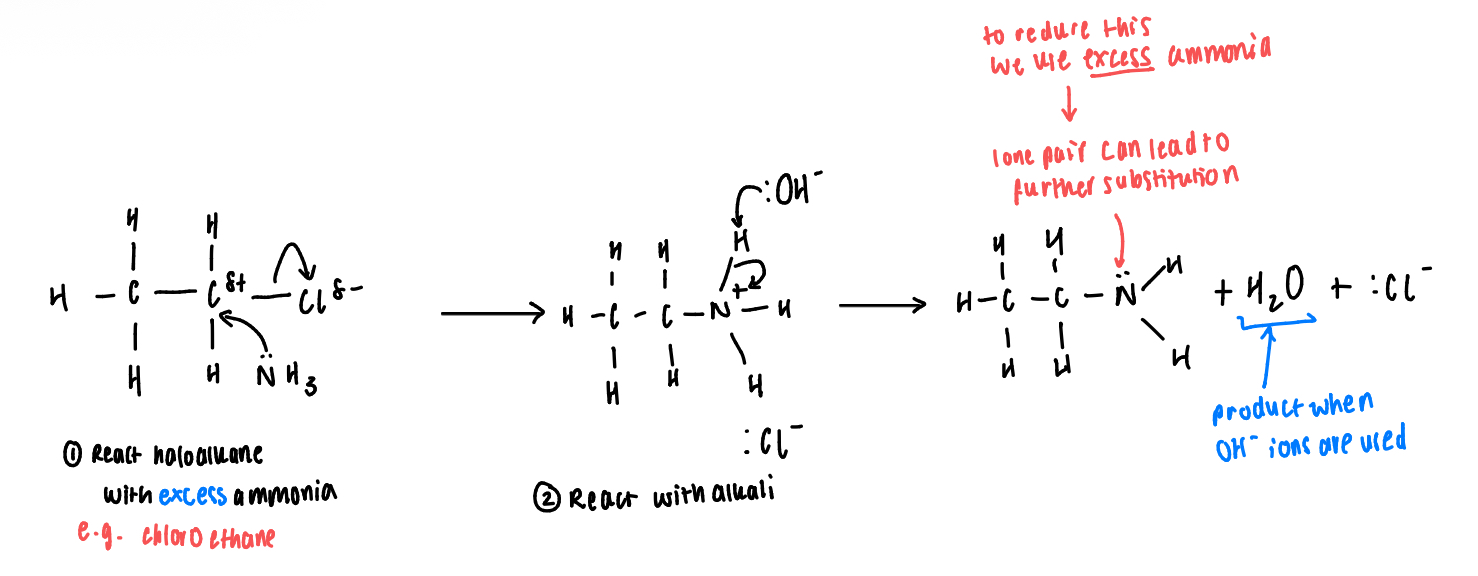
Explain in brief the preparation of aliphatic amines (conditions)
Haloalkanes are warmed gently with excess ammonia with ethanol as a solvent
Reaction performed in a sealed flask
Explain how primary amines are formed:
Ammonia has a _____ _____ ____ ______ on the ______ atom, which allows ammonia to act as a ______ in a _____ reaction with a ______
This produces an ______ __
_____ _____ is then added to generate the ____ from the salt
Ammonia has a lone pair of electrons on the nitrogen atom, which allows ammonia to act as a nucleophile in a substitution reaction with a haloalkane
This produces an ammonium salt
Aqueous alkali is then added to generate the amine from the salt
Give the equations for the formation of propylamine from 1-chloropropane
Salt formation
CH3CH2CH2Cl + NH3 → CH3CH2CH2NH3+Cl-
1-chloropropane + ammonia → propylammonium chloride salt
Amine formation
CH3CH2CH2NH3+Cl- + NaOH → CH3CH2CH2NH2 + NaCl + H2O
Propylammonium chlordide + sodium hydroxide → propylamine + sodium chloride + water
Give the essential conditions for the formation of aliphatic amines
Ethanol as a solvent
Prevents any substitution of the haloalkane by water to produce alcohols
Excess ammonia
Reduces further substitution of the amine group to form secondary and tertiary amines
Why is the reaction for formation of primary amines different from secondary and tertiary amines?
The product from the formation of primary amines still contains a lone pair of electrons on the nitrogen atom that can react further with a haloalkane to form a secondary amine, and then a tertiary amine
Show how dipropylamine is prepared from propylamine
Making an ammonium salt
CH3CH2CH2Cl + CH3CH2CH2NH2 → (CH3CH2CH2)2NH2+Cl-
1-chloropropane + propylamine → dipropylammonium chloride salt
React with alkali to obtain product
(CH3CH2CH2)2NH2+Cl- + NaOH → (CH3CH2CH2)2NH + NaCl + H2O
DIpropylammonium chloride + sodium hydroxide → dipropylamine + sodium chloride + water
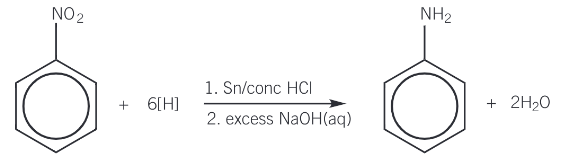
Give the conditions needed for preparing aromatic amines
Tin catalyst
Reflux (reduction)
Concentrated HCl
How is phenylamine (C6H5NH2) made?
Reduction of nitrobenzene
Nitrobenzene is heated under reflux with tin and hydrochloric acid to form the ammonium salt, phenylammonium chloride)
Tin and HCl act as a reducing agent
Reaction with excess sodium hydroxide
Reaction with sodium hydroxide forms the aromatic amine, phenylamine
Show the mechanism for the formation of a primary amine (e.g. ethylamine)