Intro to neuroscience
1/49
Earn XP
Description and Tags
- Neurones - Myelination - myelination disorders - Glial cells - astrocytes
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
50 Terms
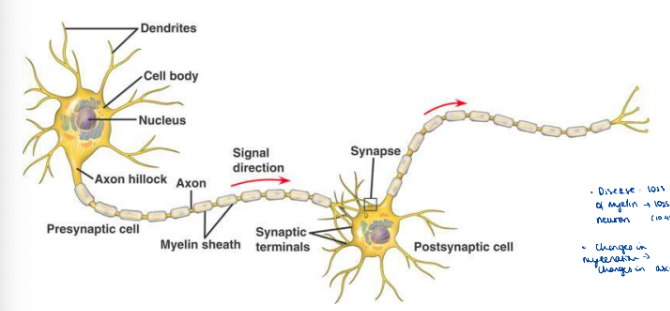
Role of dendrite
Recieve electrical + chemical messages from other neurons
Role of cell body
Process incoming + outgoing signals
Axons
Send signals to axon terminals
What is glia/glial cells? What are 2 types of myelinating glia?
Contribute to brain function mainly by insulating, supporting, nourishing neighbouring neurons
Oligodendroglial + Schwann cells
Axon terminals
Transmits signals to nearby cells
What is the resting membrane potential?
Approx -65mV
How is a resting membrane potential maintained? (5)
More K+ inside neurone, less Na+ outside CM.
Na+/K+ATPase - 3Na+ out, 2K+ in
Inside of neurone slightly negative compared to outside
Leak channels - more permeable to K+ so more K+ leaves
Energy-dependent process
Order of an action potential (graph) and what each one is caused by in terms of ion flow (4)
Resting state - no ions move through VGC
Depolarisation - by Na+ into cell
Repolarisation - by K+ out of cell
Hyperpolarisation - by K+ continuing to leave cell
What 2 types of potentials can be caused by a membrane potental?
Graded potentials (local changes in membrane potential that degrade with distance)
Action potentials (brief spike-change in membrane potential of a set amplitude and does not degrade, allows comm betwen neurons)
What do graded potentials determine?
Excitated neuron - generate AP
Inhibited neuron - less likely generate AP (at axon initial segment)
Roles of myelination + myelin sheath gaps
Saltatory conduction - fast transmission of APs
Myelin sheath - fast conduction, keeps current in axons. (voltage doesn’t decay much). APs are only generated in myelin sheath gaps, jump from gap to gap
Coordinated movement
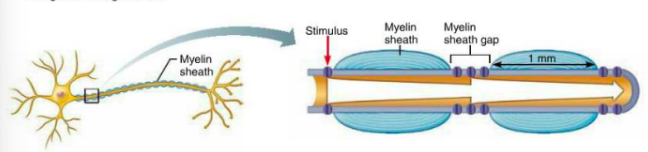
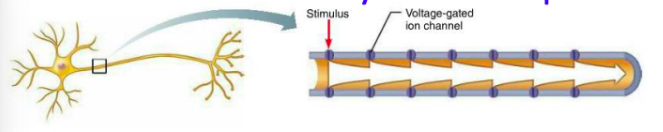
What is conduction like in nonmyelinated axons (compared to myelinated)?
Continuous conduction (slow) - takes time for ions + gates to move, must occur before voltage can be regenerated
Voltage-gated Na+ + K+ channels regenerate APs at each point along axon so voltage does not decay
When are axons myelinated
Perinatal period (before birth)
Continues into adulthood, same axons, continued proliferation, differentiation + re-modelled
What are nodes of ranvier (3)
Unmyelinated gaps, exposes neurone membrane to external environment
Gaps have lots of Na+ & K+ VGCs
APs generated by one NOR, jumps to and is regenerated to the next one - rapid transmission
Approx how wide are nodes of ranvier?
1μm wide
What is coordinated movement in childhod?
Babies develop skills to move, continue to learn new skills as grow older
Between CNS + peripheral system (listen)
How does our brain learn new skills?
Changes in number + quality of contact between neurons (synapses)
Change in myelination (ability to make new oligodendrocytes + myelin important for learning)
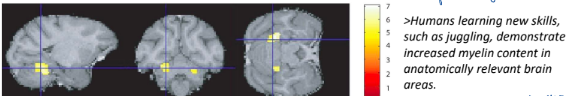
What gene is important for making new oligodendrocytes? What did an experiment prove about this gene in the ability to learn new skills?
Myrf gene (myelin regulatory factor), affects ability to perform complex tasks
Experiment:
Knockdown of Myrf gene in mice - less efficient ability to run

Alongside new myelin, what does extensive re-modelling of existing myelin affect? (3)
Compaction of the myelin sheath + length of node of Ranvier
Spatial learning lengthens node of Ranvier
Modifying the axon-glial configuration may be a mechanism that facilitates learning (in adult mouse brain)
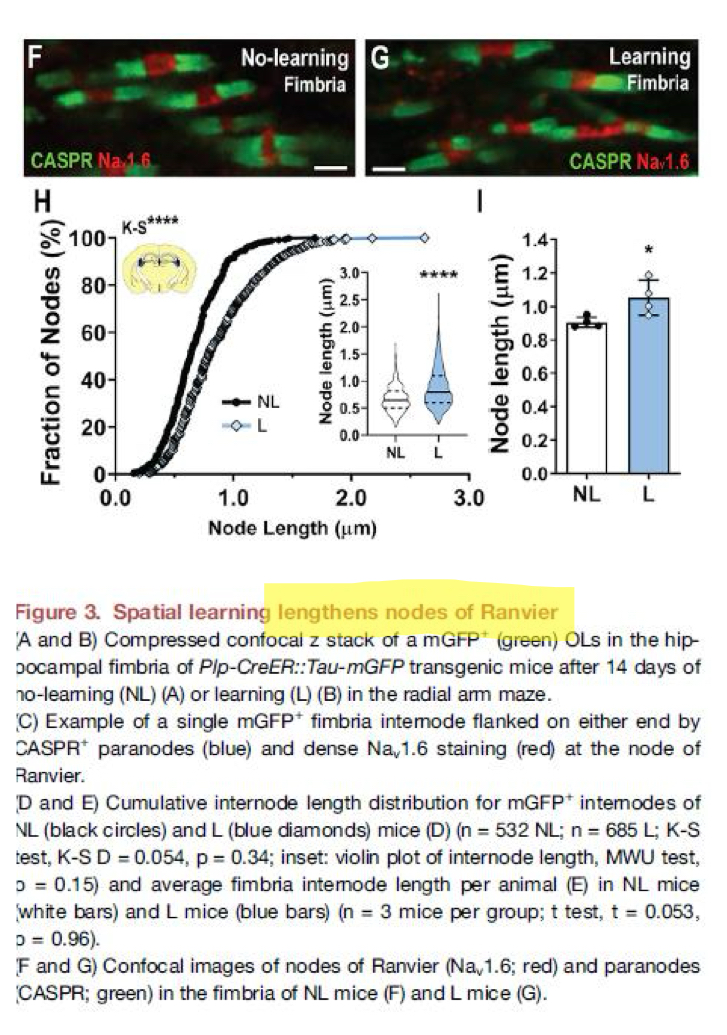
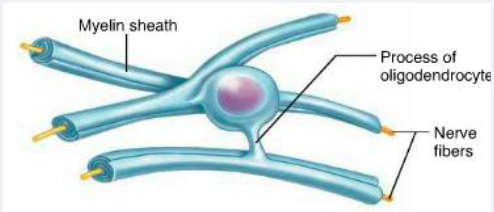
Schwann cell vs oligodendrocyte cell in making myelin
1 schwann cell makes 1 segment of myelin in PNS
1 (cell body of) oligodendrocyte can myelinate many tens of axons at the same time, in CNS
Why is more efficient for an oligodendrocyte cell body to myelinate multiple cells at the same time in the CNS?
listen to vid (18.20)
What is the node of Ranvier? What is found in the axonal membrane of NoR?
Naked (unmyelinated) bits of axon where APs are regenerated from 1 NoR to another
High conc. of VGC Na+ channels in axonal membrane
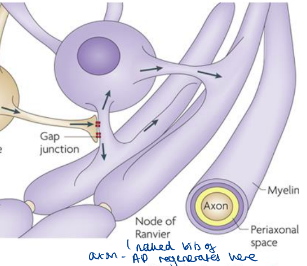
What is formed at the paranodal junction to limit the current leak from the node of Ranvier? (2)
Ends of each successive wrap of myelin form a specialised tight junction with the axonal membrane at the paranodal junction to limit the curent leak
Tight seal stops the flow of K+ in that direction (lateral diffusion/leakage away from nodal region)
How does the tight seal help with learning?
It seems to change as we learn
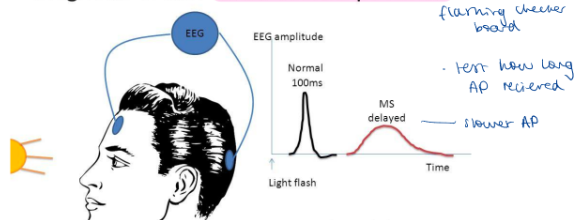
How have potential tests been used to detect inability to conduct APs caused by damage eg demyelination?
Measure electrical activity of the brain in response to stimulation
What is multiple sclerosis and how can it be diagnosed using electrical brain activity?
Multiple sclerosis:
Neurological disorder - lack of coordination, impaired vision + speech. Attacks myelin sheath of bundles of axons in brain, spinal cord + optic nerves.
Diagnosis (other than MRI scans)
Visual evoked potential
Eg stimulate eye with checkerboard pattern and measure time of electrical response
Profound slowing of conduction velocity and block in some fibres
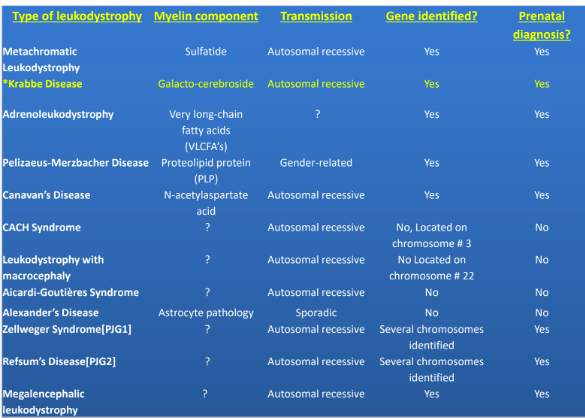
What are leukodystrophies and their characteristics? (5)
Dysmyelinating disorders with:
Primary lesion of myelin/oligodendrocyte (diseases of OD + myelin)
Genetic causes
Progressive clinical course
Predominant + confluent involvement of CNS white matter
What is a dysmyelinating disease, according to Poser 1957?
Heredofamilial disorders (genetic) where myelin not formed properly, or myelin formation is delayed/arrested, or disturbed maintenance of already formed myelin
Example of a leukodystrophy disease
Krabbe disease
What is Krabbe disease (aka globoid cell leukodystrophy)?
Autosomal-recessive condition, metabolic disorder, from deficiency of enzyme GALC (galactocerebrosidase)
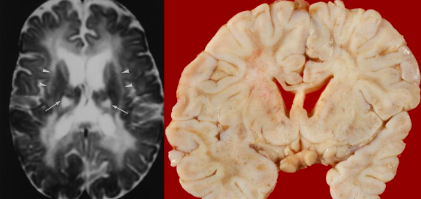
What is Krabbe disease characterised by?
Rapid + nearly complete disappearance of myelin + myelin-forming cells from the CNS & PNS
Reactive astrocytic gliosis (hypertrophy, glial scar formation, signalling molecules released)
Infiltration of the unique and often multinucleated macrophages ("globoid cells“ – green arrow)
When do sympyoms of Krabbe disease develop normally and when does it usually result in death?
Before 6 months of age, results in death by 2yrs age
What is GALC (galactocerebrosidase) responsible for? How can it be toxic to OD and other cells?
Liposomal hydrolysis of galactolipids formed during white matter myelination
It catabolises galactosylsphingosine → sphingosine and galactose
Accumulation of galactosylsphingosine is hugely toxic to oligodendrocytes and other cells.
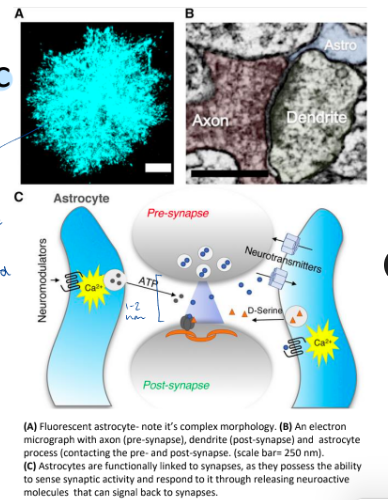
What is an astrocyte?
Type of glial cell that regulates BBB, coupling neuronal activity with metabolic support from blood, and interacts with other types of cells:
Oligodendrocytes, microglia, blood vessels
Wraps around every blood vessel + capillary
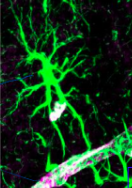
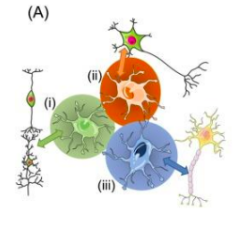
Specialised astrocytes express distinct molecular signatures (the diff colours) to do what 3 roles?
Modulate synaptic activity to clear neutrotransmitters (eg glutamate)
Release gliotransmitters
Provide metabolic support
Why + how do astrocytes interact with oligodendrocytes?
To support axon and regulate myelination process, provide atrophic supporting factors - eg molecular signals, GFs, signalling molecules)
To regulate homeostasis of brain tissues
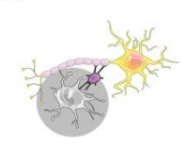
Why + how do astrocytes interact with microglia?
To critically modulate their ability to support neurons during inflammation
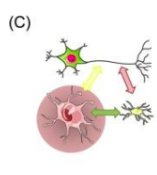
How do astrocytes help synaptic transmission?
Regulates chemical content of extracellular space. Has proteins in membranes to remove + recycle excess released neurotransmitter, hands it back to cell.
Eg tightly controls conc. of K+ in extracellular fluid
Can sense synaptic activity and respond to it by releasing neuroactive molecules that can signal back to synapses
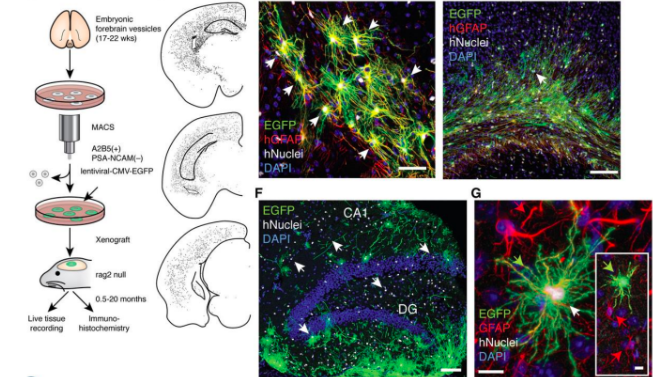
How has a study suggested that astrocytes affect learning and memory?
Human astrocytes - larger + more morphologically complex than rodent astrocytes
Transplant (hu)Astrocytes into the mouse brain and compared to controls
Improved synaptic function and mice are ‘smarter’
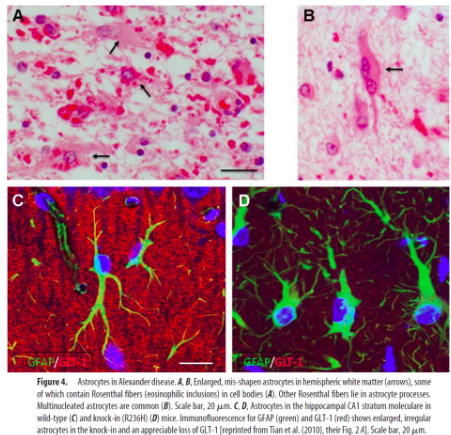
What is Alexander disease + what is it caused by?
Disease of astrocytes
A leukodystrophy characterised by abnormal protein deposits - Rosenthal fibres
Caused by dominant gain-of-function mutations in the glial fibrillary acidic protein (GFAP, expressed only in astrocytes).
Symptoms of Alexander disease
Enlarged brain and head
Seizures
Stiffness in the arms and/or legs
Learning difficulties
Delayed physical development.
Most cases begin before 2 years old (infantile form)
Most children with the infantile form do not survive beyond 6yrs
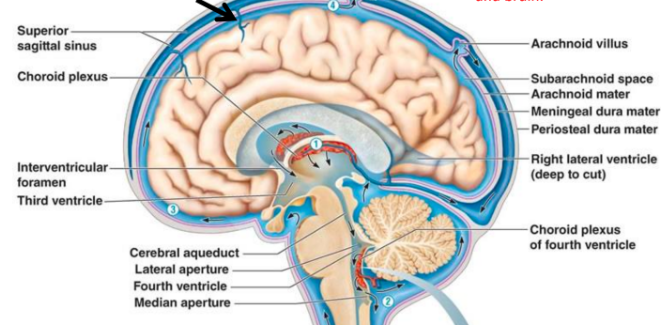
Cerebospinal fluid role
Bathes brain + spinal chord
Rich in nutrients, carries metabolites, helps clear waste products
What are ependymal cells? What do they include and what are they important for?
Line the CSF-filled ventricles of brain + spinal chord to provide physical barrier between CSF and brain
Important for CNS development
Like epithelial cells and have a basement membrane, cell-cell junctions and motile cilia on the ventricles
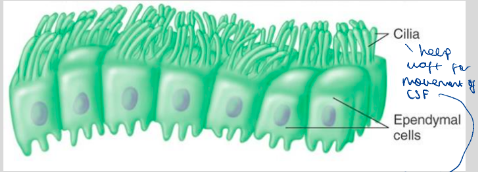
Role of cilia
Wafts to help circulate CSF around ventricles + over brain
Otherwise CSF will build up in brain → disorders
What is the choroid plexus?
In ventricles of brain, produce CSF
Formed from specialised ependymal cells
Have lots of blood vessels to filter substances (eg nutrients, electrolytes) from blood into CSF
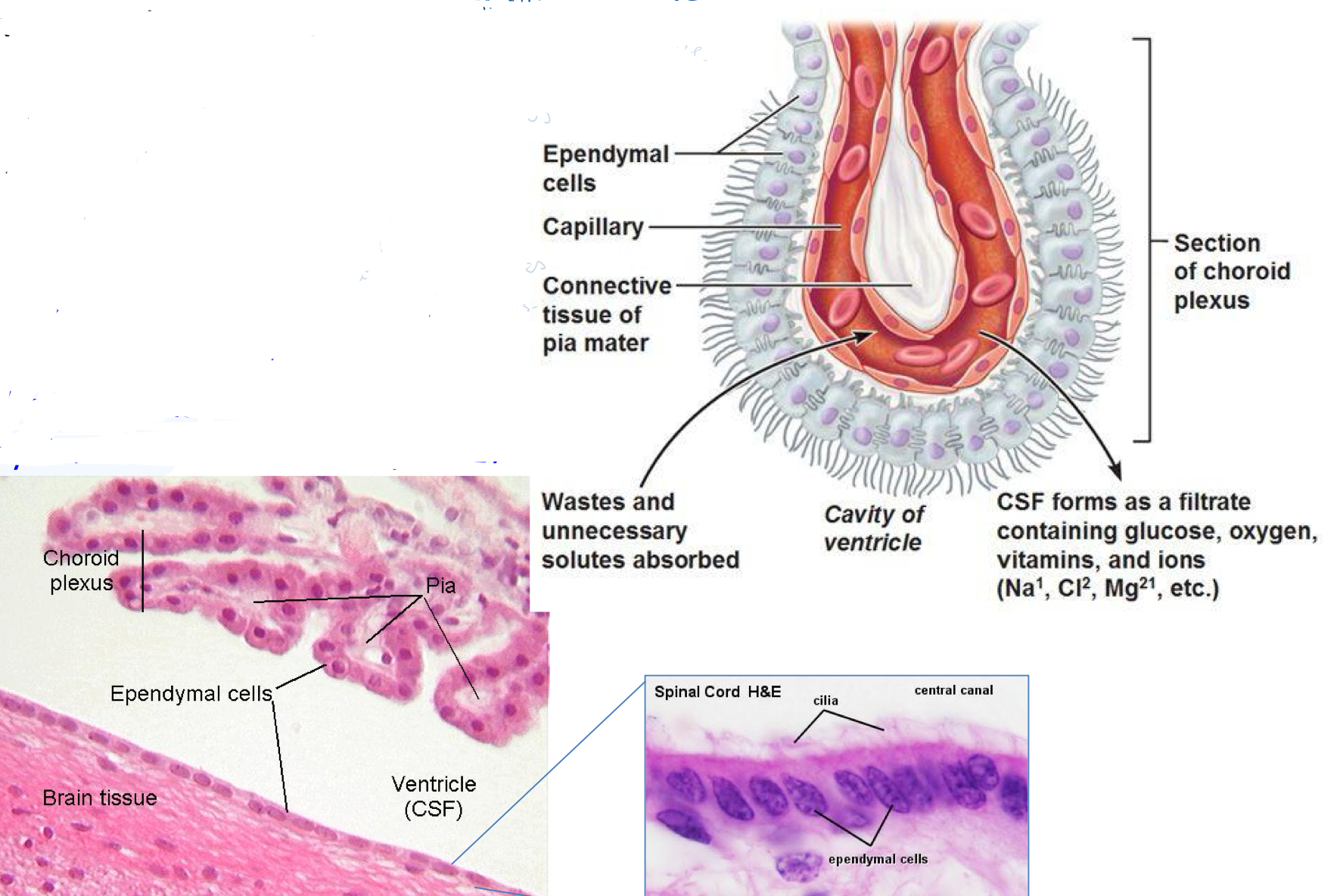
What can a choroid plexus tumour or dysfunction in cilia motility (rare) lead to?
Hydrocephalus (water on the brain)
Hyperproduction or buildup of CSF fluid → ventricles swell
Brain tissue squeezes and loses consciousness
What are microglia (2) and their roles (2) in defending the CNS?
Resident tissue macrophage of CNS, 15% of brain cells
Primary defense of CNS - first cells to respond to injury/infection
Roles:
Remove cellular debris (general maintenance + surveillance)
Neuroprotection - trophic support for neurones
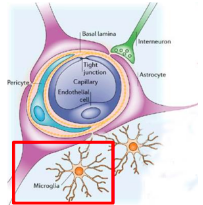
What does dysfunctional microglia lead to?
Disurpts normal brain homeostasis
Neurotoxic - neurodegeneration
Common variants in microglial-experssed genes linked to increased risk of Alzheimer’s
As microglia are reactive to changes in the brain (they proliferate and found in high numbers), what can they be used for?
Useful indicator of brain health, disease + response to treatment
What does PET (radioactive) imaging reveal about microglial density?
Indicates their activation
Expression of TSPO (translocator protein) on mitochondrial membranes of
microglia (and other cells)
Radioligands, such as [11C]PK11195, report areas of increased microglial density
![<ul><li><p>Indicates their activation</p></li><li><p>Expression of TSPO (translocator protein) on mitochondrial membranes of</p><p>microglia (and other cells)</p></li><li><p>Radioligands, such as [11C]PK11195, report areas of increased microglial density</p></li></ul>](https://knowt-user-attachments.s3.amazonaws.com/9504413d-aec5-4d6a-98e3-d490c6b2f8d8.jpeg)