9.7 galvanic (voltaic) and electrolytic cells (ap chem)
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
components of electrochemical cell
electrodes, solutions in the half cells, salt bridge, voltage/current measuring device
electrodes
solid metals connected to an external circuit that provides an electrical connection between the two parts of the system
anode
where the oxidation half-reaction occurs
cathode
where the reduction half-reaction occurs
galvanic (voltaic) cell
uses the energy released during a spontaneous redox reaction (ΔG < 0) to generate electricity
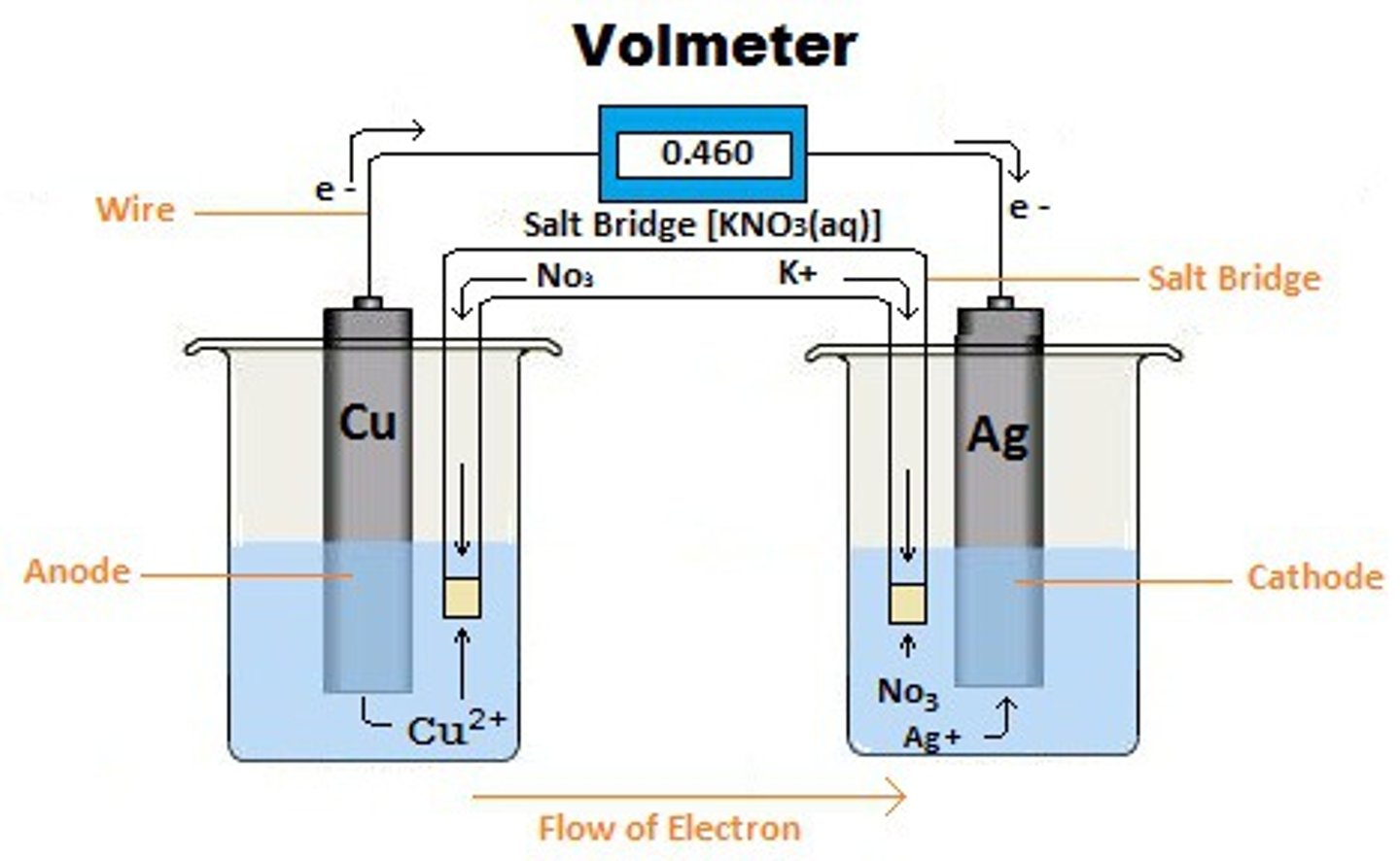
electrolytic cell
consumes electrical energy from an external source, using it to cause a non-spontaneous redox reaction to occur (ΔG > 0)
electrodes are connected by...
an electrolyte
electrolyte
an ionic substance or solution that allows ions to transfer between the electrode compartments
salt bridge
U-shaped tube inserted into both solutions that contains a concentrated liquid or gelled electrolyte
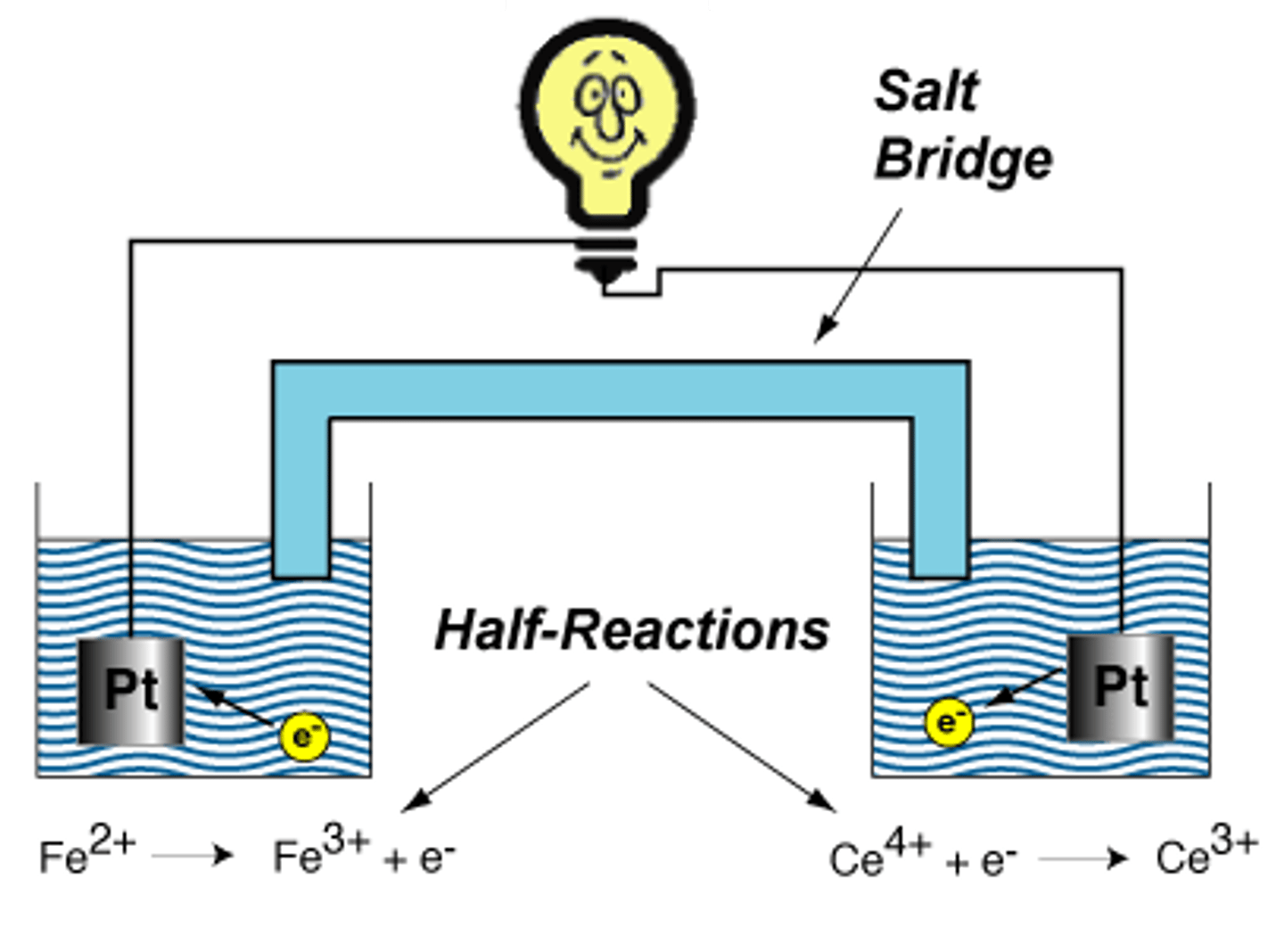
solutions in the half cells
Ionic compounds that conduct current and/or participate in the redox reactions
voltmeter
a device used to measure voltage
direction of electron flow
anode (-) to cathode (+)
electrolytic cells involve...
a thermodynamically unfavored reaction