UNIT 3
1/214
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
215 Terms
Oxidation and reduction
Oxidation is the process where electrons are lost
reduction is the process where electrons are gained
Oxidising agent
A substance that takes electrons from another substance and so it is reduced
Reducing agent
A substance that gives electrons to another substance and so it is oxidized
Oxidation number
of an element is 0
in a neutral compound add to 0
in a charged compound adds to total charge eg. +1
Hydrogen is +1 (except metal hydrides = -1)
oxygen is -2 ( except peroxides and F2O = -1)
Halogens are -1
Group 1 are +1
Electrochemical cell diagram
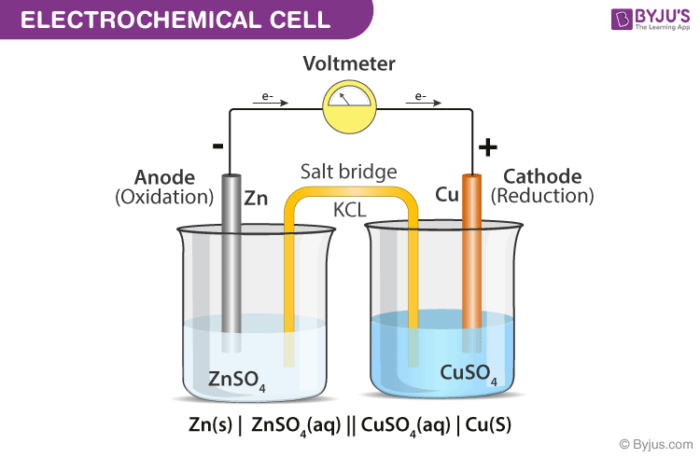
Purpose of the wire in the half cell
O-R
Allows electrons to flow from the half cell where oxidation occurs to the half cell we're reduction occurs i
ncluding a high-resistant voltmeter if we are measuring the potential difference in the cell
Purpose of the salt bridge in the half cell
completes
Complete the circuit and allows ions to flow without the Solutions mixing
typical salt bridge is made of a gel soaked in a solution of potassium nitrate
The half cell of a metal/ metal ions
we would have a piece of metal as the electrode with a solution containing 1mol dm-3 solution of metal ions
A half cell with a gas in contact with a solution of non-metal ions with an inert metal electrode
Non-metals are not conductors so we must use an inert platinum electrode to allow electrons to flow in or out of the half cell
typically used for a hydrogen electrode or oxygen half cells
the gas is bubbled over the inert electrode which is dipping into a solution of ions the changes do not cause any apparent colour change
Half cell with a solution containing ions of a metal into different oxidation states, using an inert metal electrode
No conductor in the system so an inert platinum electrode is used to allow electrons to flow in or out of the half cell. Typically used for transition metals where the metal may have several oxidations States eg. Fe2+/ Fe3+ and Mn2+/MnO4-
both of these half cells cause color changes when oxidation or reduction occurs
Representing half cells
Mg(s) | Mg2+ (aq) - this is the metal/ metal ion half cell
Pt (s) | H2(g) | H+ (aq) - gas/solution half cell containing in it platinum electrode
Pt(s)| Mn2+(aq), MnO4- (aq) - mixed solution half cell containing inert platinum electrode
Standard electrode potential
The ability of a half cell to gain or lose electrons is measured using standard electrod potential E©
measured in volts
species that are easier to reduce have a positive e-value
species that are easier to oxidize have a negative e-value
Standard hydrogen electrode
Consists of a platinum electrode dipped in 1.0 mol dm^-3 solution of H+, used as hydrochloric acid and hydrogen gas is slowly passed over the electrod at a pressure of 1 atm and a temperature of 298K
the standard electrode potential for this half cell is taken as 0.00 V
Why is platinum foil used
Used as an electrode hydrogen isn't an electrical conductor so platinum is inert and provides electrical conduction
used when the half cell doesn't contain a metal
To measure the standard electoral potential
writing
We must set up a half cell under standard conditions and connect it to a standard hydrogen electrode
if two half cells are combined we must use a double line between them to represent a salt Bridge and place the metals at both ends
a high resistant voltmeter is connected between the electrical conductors of both electrodes
Using the standard electrod potentials- negative value
Reading on voltmeter gives E value
a negative signifies that the electrode potential is more negative than the potential of these standard hydrogen electrode, means electrons must be flowing along the wire from the half cell to the standard hydrogen electrode and the hydrogen electrode becomes the positive electrode
Using the Standard electrode potential- positive value
The positive signifies that the standard hydrogen electrode has more negative potential
electrons therefore flow along the wire from the standard hydrogen electrode to the half cell and the hydrogen electrode becomes the negative electrode
Electoral chemical series
the most reactive metals
non metals
The most reactive metals have the most negative E value the most reactive non-metals have the most positive E value
the strongest oxidizing agents are those that have the most positive standard electrode potentials the
strongest reducing agents are present in the half equations with the most negative standard electrode potentials
Calculating the EMF of an electrochemical cell
Given by the difference between the standard electrode potentials of two half cells. Value is positive
EMF= (Rhs) - (Lhs)
Feasibility of reactions
In general if the overall cell potential is positive the reaction taking place is spontaneous and more favorable
Anticlockwise rule
Write the two half equations with the most negative value on top
by drawing anti-clockwise arrows means that in the feasible reaction the top reaction goes in reverse and the bottom goes in the direction is written
balance the electrons and equation
write out the balanced cell equation
Calculating the EMF of SEP reaction
= reduction- oxidation
the stronger oxidizing agent will have a more positive value
if the value is negative the reaction is not feasible
Le chateliers principal
State the position of equilibrium of a system changes to minimize the effect of any change in conditions
Temperature and cell potential
If the cell reaction as written is exothermic then as the temperature increases the position of equilibrium shifts to the left, meaning the cell potential becomes more negative
Concentration and cell potential
If the concentration of the reactions are increased then the position of equilibrium shifts to the right and the cell potential becomes more positive
if the concentration of one of the products increases an opposite effect is observed and the potential becomes more negative
Electrode potential and pressure
A change in pressure only affects those reactions that involve the gas
the effect is the same as concentration
Fuel cells
electrochemical
An electrochemical method of releasing energy which avoids the need to burn the fuel followed by using this heat to cause an expansion which is used to move a motor
energy is lost at each stage of the traditional process with much of the energy released from the fuel being lost as heat out of the exhaust
Fuel cell system
passes fuel
protons diffuse thru
electrons removed form a
The fuel cell system passes the fuel over platinum metal which acts as a catalyst but also as an electrode for the electrochemical system.
Electrons are removed from hydrogen atoms at one electrode.
H2 → 2H+ + 2e-
the protons diffuse through a semi permeable membrane to the other electrode where they receive electrons and oxygen molecules to form water molecules
O2 + 4H+ + 4e- → 2H2O
overall reaction is ( 2H2 + O2 → 2H2O) and voltage produced is 1.23V
Benefits of fuel cells
They are a convenient way of storing and releasing energy
energy efficiency is much higher than standard fuel systems
emissions from fuel cells are less damaging than the carbon dioxide from traditional engines
Drawbacks of fuel cells
conversion process
Hydrogen fuel must be generated Elsewhere and this is likely to use fossil fuel energy sources which will cause their own carbon dioxide emissions
also an energy loss here as the conversion process is not 100% efficient
the gas is needed are difficult to store compared to liquid fuels
the fuel cells operate at lower temperatures so need very efficient catalysts which use expensive metals
ion/ half equations
Elements where the oxidation state of a single atom is changing only requires electrons to balance
eg. Mg→ Mg2+ + 2e-, Fe3+ +e-→ Fe2+
compound ions require oxygen, water, hydrogen and electrons to balance
eg. MnO4- + 8H+ +5e-→ Mn2+ + 4H2O
Combining half equations
If needed multiply equations so that the electrons are balanced
cancel out electrons on both sides add elements if match
can simplify if you can
Redox titrations
involves 25cm3
Involves 25cm3 of solution measured using a volume metric pipette and placed in a conical flask. Second solution is added a little at a time from burette swirling the mixture during addition. Continued until desired color change is seen.
Many redox titrations do not need an indicator as colours of reactants frequently allow the endpoint to be seen
Acidified Manganate (VII) ions with iron (II) ions
purple sol of
burette
Purple solution of oxidizing agent is added from the burette. When it reacts with species to be oxidized it forms Mn2+ (colourless). The Ependpoint is reached when solution goes pale pink, this is because a very small amount of purple manganate remains appearing pink when dilute.
MnO4- + 8H+ +5e- → Mn2+ + 4H2O
the colour change of solution Fe2+ (pale green) is oxidised to Fe3+ (yellow) is usually difficult to see a solutions are dilute colour change associated with manganate is used for endpoint. iron ions are oxidized:
Fe2+ → Fe3+ + e-
Acidified dichromate (VI) ions with iron (II) ions
in acid
Potassium dichromate in acid solution oxidizes Fe2+ to Fe3+ with a color change from Dark orange Cr2O72- to green solution of Cr3+
Cr2O72- + 14H+ +6e- → 2Cr3+ + 7H2O
iron is again oxidized
Fe2+ → Fe3+ + e-
Acid in the dichromate titration
if the ph of solutions
COLOUR change
Acid must be present for this reaction to occur usually sulfuric acid
if the pH of a solution Rises too high then dichromate ion is broken up into two chromate ions:
Cr2O72- + H2O = 2CrO42- + 2H+
dark orange. Yellow
chromium always has an oxidation state of plus six so no redox reactionchromium always has an oxidation state of +6 so no redox reaction
Equilibrium if acid is added
Equilibrium will shift to the left and to the right if base is added
Redox titration for copper (II) ions
Addition of colourless solution containing iodide ions to a blue solution of copper (II) ions lead to the formation of a cloudy brown solution. Cu2+ ions in solution react with iodide ions generating a brown solution of iodine and are reduced to Copper (I) in the precipitate of CuI.
2Cu2+ +4I- →2CuI + I2
iodine is titrated with Sodium thiosulfate to work out how much copper was present originally. Sodium thiosulfate is a common reducing agent.
2S2O32- → S4O62- + 2e-
I2 +2e- = 2I-
Brown. Colourless
Experiment details
adding excess iodide ions
titrate bbrown
adding a start indicator
Adding excess iodide ions to Cu2+ ensures all Cu2+ reacts to produce I2. Mixture formed is a cloudy Brown solution
titrate the brown I2 against sodium thiosulfate until mixture is straw coloured
adding a start indicator goes blue black and continue until colour vanishes
Finding the molar mass of a redox titration
Find out the number of moles
workout in either 25cm3 or 250cm3
ratio between compounds
work out the other moles
workout the concentration then the molar mass
Oxidation states
Elements in the P block typically have two oxidation states
the maximum oxidation state which equals the group number and a lower oxidation state which is two less
as you go down the group the trend decreases
How does carbon display a + 4 oxidation state
An electron is promoted and excited from 2s orbitals to 2p
2s orbital is now unpaid and four orbitals are now hybridized in the same energy level
Inert pair effect
amount of energy returned
The tendency of S2 pair of electrons in an atom to stay paid leading to a lower oxidation state
As you go down the group the amount of energy “returned” when bonds are formed decreases. This means insufficient energy is available for the promotion of S electrons and so they stay paired
As you go down the grou
Atomic radius increases
electronegativity decreases
less covalent bonding
increase metallic character
As you go across the group
Atomic radius decreases
electronegativity increases
more covalent bondingl
less metallic character
As you go diagonally across the group
Atomic radius increases
electronegativity increases
Tin (II)
Lead (IV)
Tin wants to be +4 oxidation state, reducing agent supplies the electrons and therefore acts as a reducing agent so it oxidized itself
Lead wants to be plus two oxidation state so receives/ gains electrons as an oxidizing agent so reduces itself
Octet expansion
The ability of some atoms to use d-orbitals to have more than eight electrons in their valence shells
limits the numbers of bonds that can be formed in the first row elements
boron conform 3 covalent bonds and is electron deficient
carbon can form 4 covalant bonds
nitrogen can form 3 covalent bonds and 1 lone pair
oxygen can form 2 covalent bonds and 2 lone pairs
Other members of the group ( 3rd period and below)
they have acess to
s orbitals promote an electron
They have access to d-orbitals that are not present in the second shell allowing them to expand their octet and every electron in the outer shell can be used to form covalent bonds
no longer a limit of eight electrons in the outer shell
the s orbitals promote an electron to the d orbitals in which singular orbitals are formed
Metallic character
elements at the top of each group are non metals eg, B, C, N
elements at the bottom of each group are metals
change in properties lead to the charactistic zigzag line between metals and non metals
Amphoteric
materials that react with both acids and bases
typically show the material reacting with an acid such as hydrochloric or nitric acid and sodium hydroxide
Reactions with aluminium oxide
HCL and NaOH
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Al2O3 + 2NaOH + 3H2O → 2Na[Al(OH)4]
Reactions with Aluminium hydroxide
H+ and OH-
Al(OH)3 + 3H+ → Al3+ + 3H2O
Al(OH)3 + OH- → [Al(OH)4] -
Reactions with lead oxide
HNO3 and NaOH
PbO + 2HNO3 → Pb(NO3)2 + H2O
PbO + 2NaOH + H2O → Na2[Pb(OH)4]
Reactions with lead Hydroxide
H+ and OH-
Pb(OH)2 + 2H+ → Pb2+ + 2H2O
Pb(OH)2 + 2OH →[Pb(OH)4]2-
Reactions with aluminium
OH-
ppt,
Solutions containing amphoteric metal compound form precipitates when sodium hydroxide is added. These precipitates are metal hydroxides. Since the hydroxide can react with more sodium hydroxide these precipitates will then redisolve
Al3+ + 3OH-(aq) → Al(OH)3(s)
Then: Al(OH)3 (s) + OH-(aq) → [Al(OH)4] -
Reaction with lead- ppt
OH-
Pb2+(aq) + 2OH-(aq) → Pb(OH)2 (s)
then: Pb(OH)2(s) + 2OH- (aq) → [Pb(OH)4]2- (aq)
Electron deficiency
An electron deficient atom is one that doesn't have a full outer shell, when elements in group 3 form compounds they commonly form three covalent bonds such as BF3, BCl3, AlCl3
To fill their outer shell, these atoms will often form coordinate bonds to gain extra electron pairs (they are electron acceptors)
AlCl3 → Al2Cl6

in this case each electron deficient aluminium atom uses a lone pair on a chlorine atom to form a coordinate bond
the aluminium chloride dimer no longer has any electron deficient atoms as each aluminium atom has eight electrons in its outer shell
other compounds that can form coordinate bonds to remove their electron deficiency our classified as doner- acceptor compounds where one molecule donates a lone pair and the other accepts it eg. BF3 and NH3
Boron Nitride
B-N bond Is similar to C-C bond
there are a total of 12 electrons on the two atoms the atomic radii of all the atoms are similar with carbon being almost exactly the average of the radii of boron and nitrogen, sharing a similar relationship in their electronegativities
boron nitride can have several forms which are similar to the several different forms of carbon
Hexagonal boron nitride- graphite structure
Can form hexagonal sheets similar to those found in graphite
in this case the atoms in different layers lie directly above one another with each boron having a nitrogen atom directly above and below it
differs from graphite as the layers in graphite are arranged so that atoms on adjoining layers are not directly above one another
forces between layers are weak so boron nitride shares ability for layers to slip over one another with graphite - used as lubricant
each carbon is covalenty bonded to 3 others
IS ISOELECTRIC
Electrical properties of boron nitride
No delocalized electrons present, with electrons localized as lone pairs on N atoms
the B-N bonds are polar due to the different electronegativities of the two atoms making BN an insulator and leads to its use in electronics as a substrate for semiconductors, microwave transparent windows.
Structural material for seals, electrodes
catalyst carriers in Fuel Cells and batteries
Cubic boron nitride- diamond structure
Like Diamond cubic boron nitride is extremely hard with a high melting point as covalent bonds must be broken to break or melt the solid
leads to its use as a resistant coating or an industrial abrasive
Group 4 chemistry
Has a range of metals and non-metals
as you go down the group The oxidation states go from +4 to +2 due to inert pair effect
carbon is a non-metal which forms huge range of covalent compounds whereas lead is a metal which forms ionic compounds, being the most stable
Carbon
Co2 is the most stable oxide of carbon. Carbon monoxide is the only stable compound to contain carbon in the +2 oxidation state
CO will act as a reducing agent as it easily becomes oxidized from +2 to +4. Carbon monoxide is used as a reducing agent especially for extracting metals from the oxides
Carbon monoxide with iron oxide and copper oxide
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
CuO (s) + CO(g) → Cu (s) + CO2 (g)
method can only be used for the oxides of the less reactive muscles the oxides of the more reactive metals are too stable and will not react eg. Anything above zinc
processes used to extract metals above sync is electrolysis
Lead
Lead (II) oxide, PbO, is the most stable oxide
Lead (IV) oxide, PbO2, will act as an oxidizing agent as it easily becomes reduced from +4 to +2 all lead compounds are oxidizing agents
PbO2 with HCl
PbO2 (s) + 4HCl (conc) → PbCl2 (s) + Cl2 (g) + 2H2O (l)
Oxides of carbon- acid base properties
Carbon dioxide is a colourless gas made up of small covalent molecules
this is an acidic oxide as the oxide is soluble
in water to give the very weak acid carbonic acid
Co2 (g) + H2O = H+ (aq) + HCO3- (aq)
Carbon dioxide reacted with alkalis
Forms a salt. All salts produced in this way are carbonates or hydrogen carbonates
CO2 (g) + 2NaOH (aq) → Na2CO3 (aq) + H2O (l)
CO2 (g) + NaOH (aq) →NaHCO3 (aq)
Oxides with lead: acid-based properties
Lead (II) oxide is an orange solid which contains bonding which is mainly ionic
Lead (II) oxide is amphoteric
acting as a base:
PbO (s) + 2HNO3 (aq) → Pb(NO3)2 (aq) + H2O (I)
acting as an acid:
PbO(s) + 2NaOH(aq) + H2O(I) → Na2[Pb(OH)4] (aq)
Carbon and silicon
liquids
Stable chlorides of carbon and silicon are tetrachlorides CCl4 and SiCl4
both colourless liquids containing individual covalent molecules
the molecules found in both are tetrahedral due to the 8 electrons in the valence cells
Reactions w Water CCl4
doesnt react
doesnt combine readily
CCl4 doesn't react with water as it forms a separate layer under the water
the carbon atom cannot combine easily with water molecules. This lack of reactivity is due to the absence of available d-orbitals in the valence shell, meaning that the octet cannot be expanded to allow the water molecules to be combined with the carbon atom
Reaction with water SiCl4
Reacts very quickly with water in a hydrolysis reaction this reaction produces fumes of hydrogen chloride gas and silicon dioxide SiO2 as a solid precipitate
the reaction becomes more vigorous down the group as the Bonds in the compound become weaker
SiCl4(l) + 2H2O(l) → SiO2(s) + 4HCl (g)
Increase reactivity in silicon tetrachloride
complex molecule
Silicone possesses available 3d orbitals in addition to the 3s and 3p orbitals involved in bonding to the chlorine atoms
the lone pairs of the water can form co-ordinate bonds with these empty d- orbitals giving a complex molecule that can then eliminate two HCl molecules
this molecule can then eliminate two more molecules of HCL leaving silicon dioxide
Reactions with water lead chloride
white
Lead (II) chloride is the most stable chloride of Lead. It is a white ionic solid made up of Pb2+ and Cl- ions as it is an ionic compound it doesn't react with water, but neither does it dissolve in cold water although it can be dissolved in hot water
Reactions of Solutions of Lead (II) compounds
naoh
excess naoh
hcl
ki
Solution added- NaOH, anions present- OH-
initial white precipitate of Pb(OH)2 is formed
Pb2+(aq) + 2OH- (aq) → Pb(OH)2 (s)
excess NaOH, OH-
white precipitate redisolves in excess hydroxide to form tetrahydroxoplumbate (II) ion
Pb(OH)2 (s) + 2OH-(aq) → [Pb(OH)4]2- (aq)
HCl, Cl-, dense white precipitate of Lead chloride
Pb2+(aq) + 2Cl- (aq) → PbCl2(s)
KI, I-. Dense bright yellow precipitate of Lead iodide
Pb2+ (aq) + 2I-(aq) → PbI2 ()
Oxidizing power of halogens
value for cl and i
readily gains
Oxidising power decreases down the group
the ability to remove electrons from other species can be measured used standard electrode potentials
The value for chlorine is the most positive it readily gains electrons to form chlorine ions. This also shows that it is difficult to oxidize chloride ions to chlorine molecules
the value for iodine is the least positive. It gains electrons less readily than bromine and chlorine, also shows that it is easier to oxidise iodide ions than it is to oxidise bromide and chloride
Reactions of concentrated sulfuric acid with sodium halides
Concentrated sulfuric acid is a strong acid and an oxidizing agent
when any sodium halide is added to concentrated sulfuric acid the hydrogen halide is formed as a steamy gas
sulfuric acid or products formed from it may oxidize the halide in the hydrogen halide to form the halogen, if the halide is relatively easy to oxidize process becomes easier lower down the group
Sodium chloride and sulfuric acid
Produces HCL gas. Hydrochloric acid is difficult to oxidize so the sulfuric acid doesn't cause any redox reactions
NaCl(s) + H2SO4(aq) → NaHSO4(s) + HCl(g)
steamy fumes of HCL
Sulfuric acid and sodium bromide
Produces HBr gas
NaBr(s) +H2SO4 (conc) → NaHSO4(s) + HBr(g)
sulfuric acid oxidizes some of the hbr to form Brown fumes of BR2 and SO2 gas. the Hydrobromic acid is slightly easier to oxidize and so sulfuric acid causes a redox reaction
2HBR(s) + H2SO4 (conc) → SO2(g) + Br2(g) +2H2O(l)
steamy films of HBr, orange fumes of BR2
Sodium iodide and sulfuric acid
Initially produces HI gas
NaI(s) + H2SO4(conc)→ NaHSO4(s) + HI(g)
sulphuric acid easily oxidizes HI
2HI(s) + H2SO4 (conc) → SO2(g) + I2(s) + 2H2O (l)
steamy fumes HI, purple fumes of I2 or black/solid Brown solution, yellow solid of S
Disproportionation reaction
One in which the same element is both oxidized and reduced forming products containing the element in two different oxidation states
Reaction with chlorine and water
When chlorine is bubbled through water a reversible reaction
Cl2(g) + H2O(l) → HCl (aq) + HOCl(aq)
chlorine is both oxidized and reduced
Reaction with chlorine and sodium hydroxide
Will force the equilibrium to the right
Cl2 + 2OH- → Cl- + OCl- + H2O
the ClO- ion is stable in the solution at room temperature
Reactions with chlorine and heated concentrated sodium hydroxide
Disproponination reaction
3Cl- + 6OH- →5Cl- + ClO-+ 3H2O
the chlorate ion
Uses of chlorine and chlorate ions
ClO-+ 2H+ + 2e- → Cl-+ H2O
ions are being reduced
chlorine is an oxidising agent: Cl2 +2e- → 2Cl
oxidising power of chlorine and of chlorate ions is used in bleachers. The oxidizing ability of CO- leads to its ability to kill bacteria as the microbes are oxidized
this is the basis of chlorination of water supplies to disinfect them
The dblock
outer electrons
Group of elements whose outer electrons are found in the d orbitals
Transition elements
metal that possess a partially filled d sub shell in its atom or stable ions
Why chromium and copper exceed the rule
4s orbitals donate an electron to the 3d orbitals, to give them a filled shell. Makes more stable configurations
it is more energetically favorable to have a full 3D shell
Why can elements form different oxidation states
energies
The energies of the 4S and 3D orbitals are very similar so the energy required to remove any of these electrons are similar. As the element form compounds, energy is released either through the formation of covalent bonds or ionic lattice. The energy needed to reach higher oxidation states and the energy released in compound formation is balanced allowing a range of oxidation states to form.
Why can't scandium and zinc exhibit variable oxidation states
Ions must be stable to exist
zinc and scandium aren't stable enough after losing electrons to exhibit other oxidation states
A ligand
An ion or molecule that donates a pair of electrons to a transition metal atom or ion, forming a coordination complex eg. H2O, NH3, Cl-
Examples of complex ions
[Fe(H2O)6]2+ : Pale green, octrahedral
[Fe(H2O)6]3+ : yellow, octrahedral
[Cu(H2O)6]2+ blue
[Cr(H2O)6]3+ : dark green, octrahedral
[Co(H2O)6]2+ : pink, octrahedral
[CuCl4]2- : yellow or green, tetrahedral
[CoCl4]2- : blue, tetrahedral
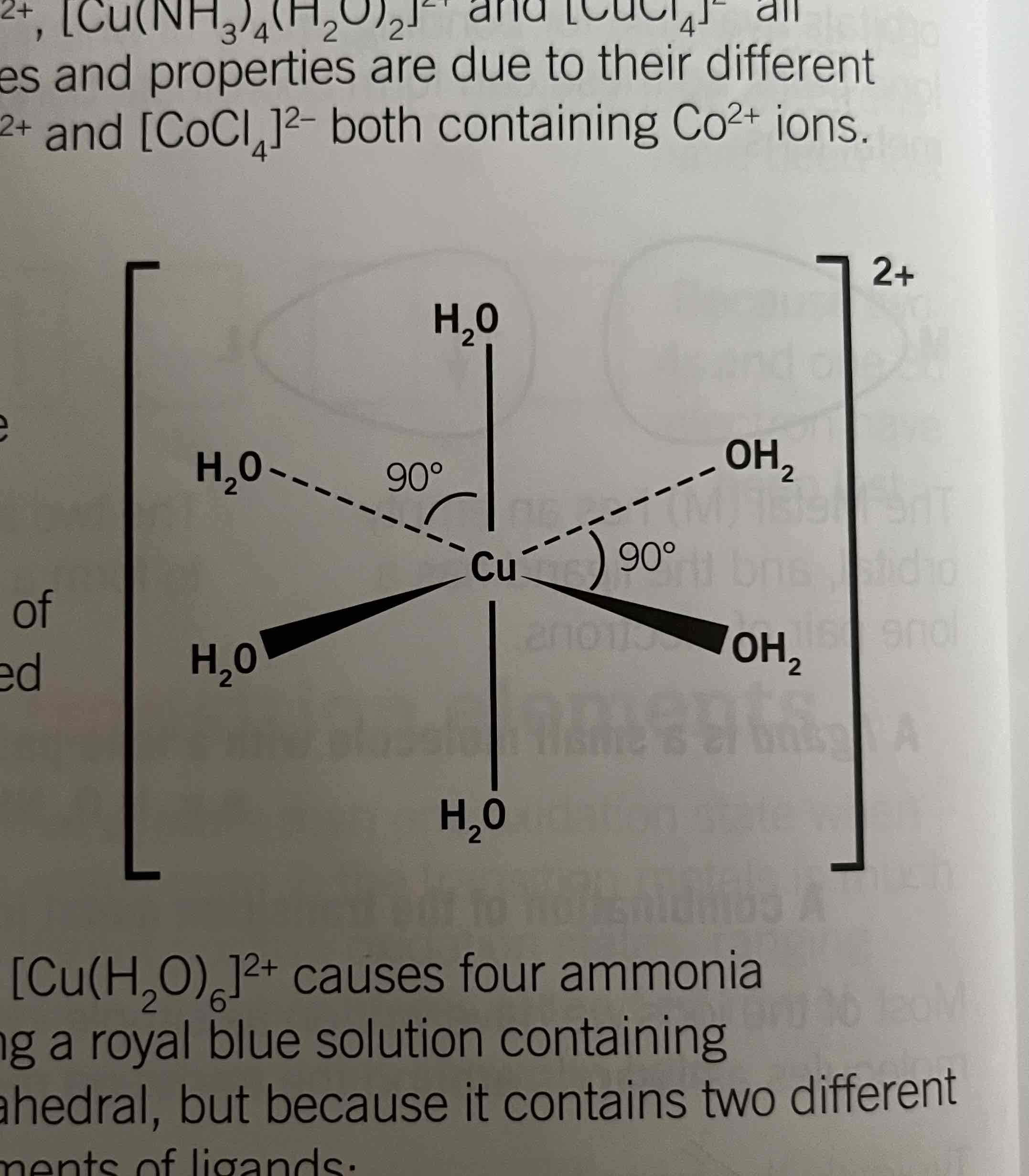
[Cu(NH3)4(H2O)2]2+ : royal blue, octrahedral
Structure of complexes: [Co(H2O)6]2+ and [Cu(H2O)6]2+
One lone pair of oxygen atoms of the water molecules are used for the bonding to the metal ion

![<p>Structure of [Cu(NH3)4(H2O)2]2+</p><p>Trans and cis isomers</p>](https://knowt-user-attachments.s3.amazonaws.com/e091453a-1e9e-4796-9ed3-bfb2e245bc0c.jpg)
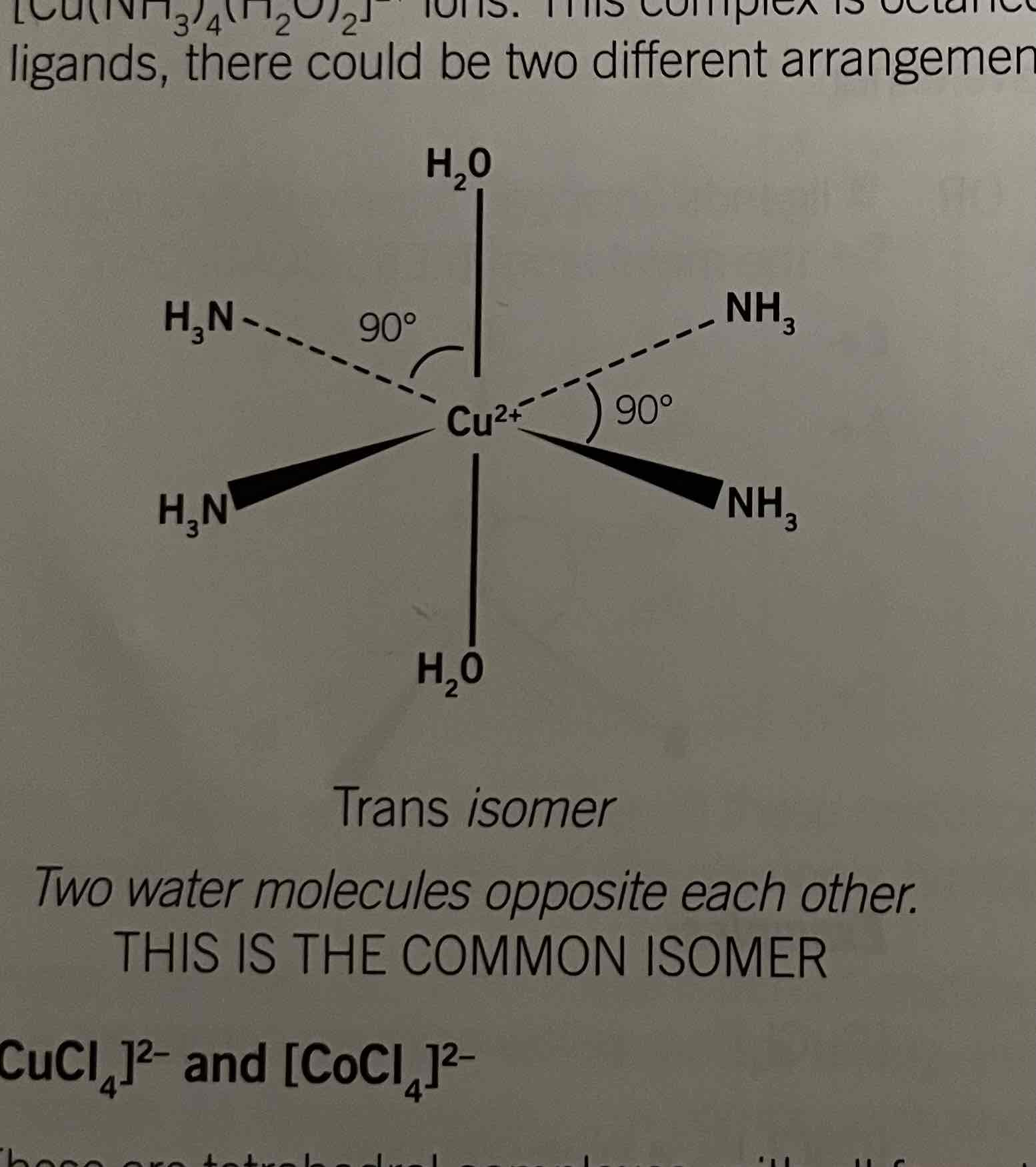
Structure of [Cu(NH3)4(H2O)2]2+
Trans and cis isomers
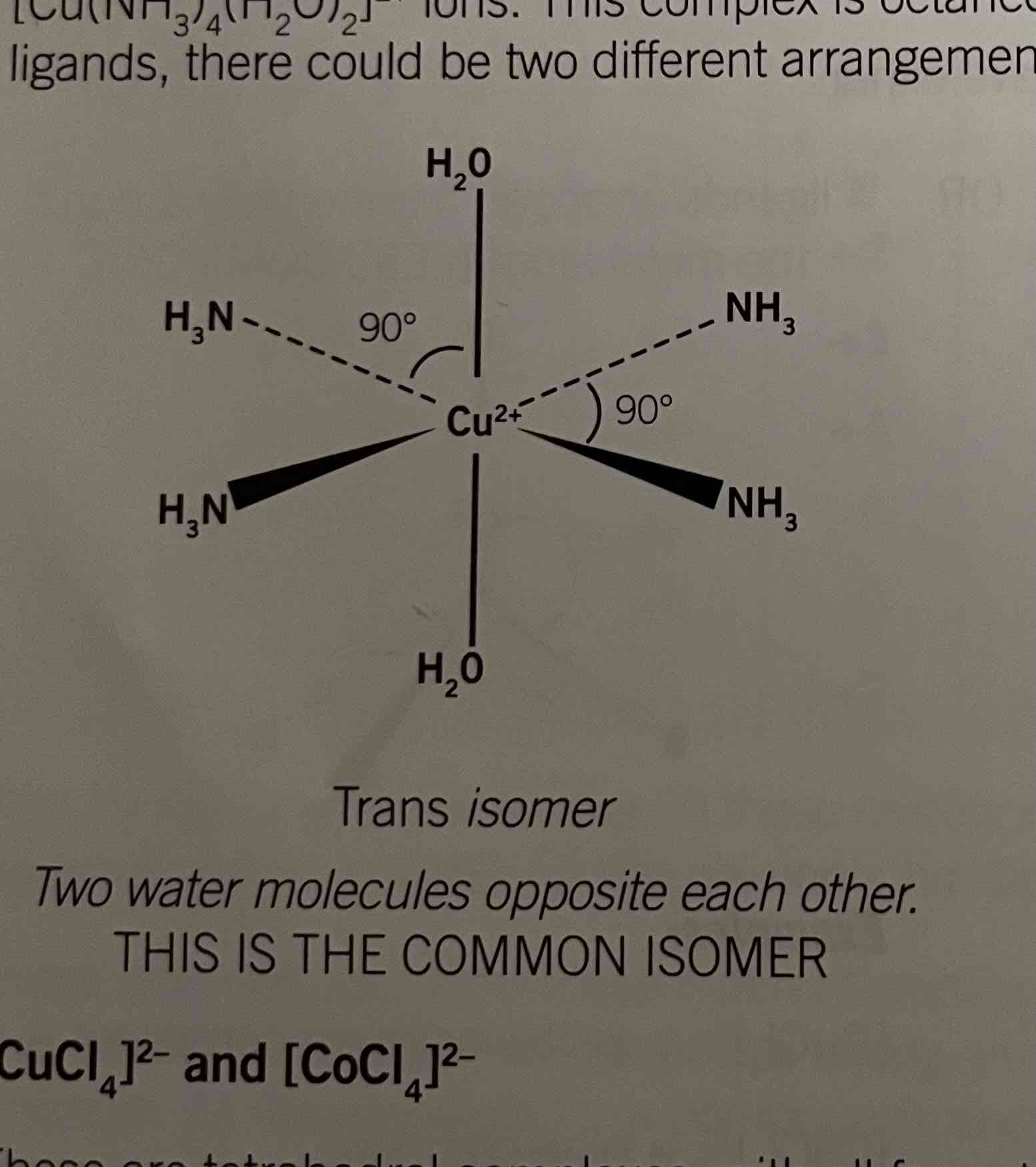
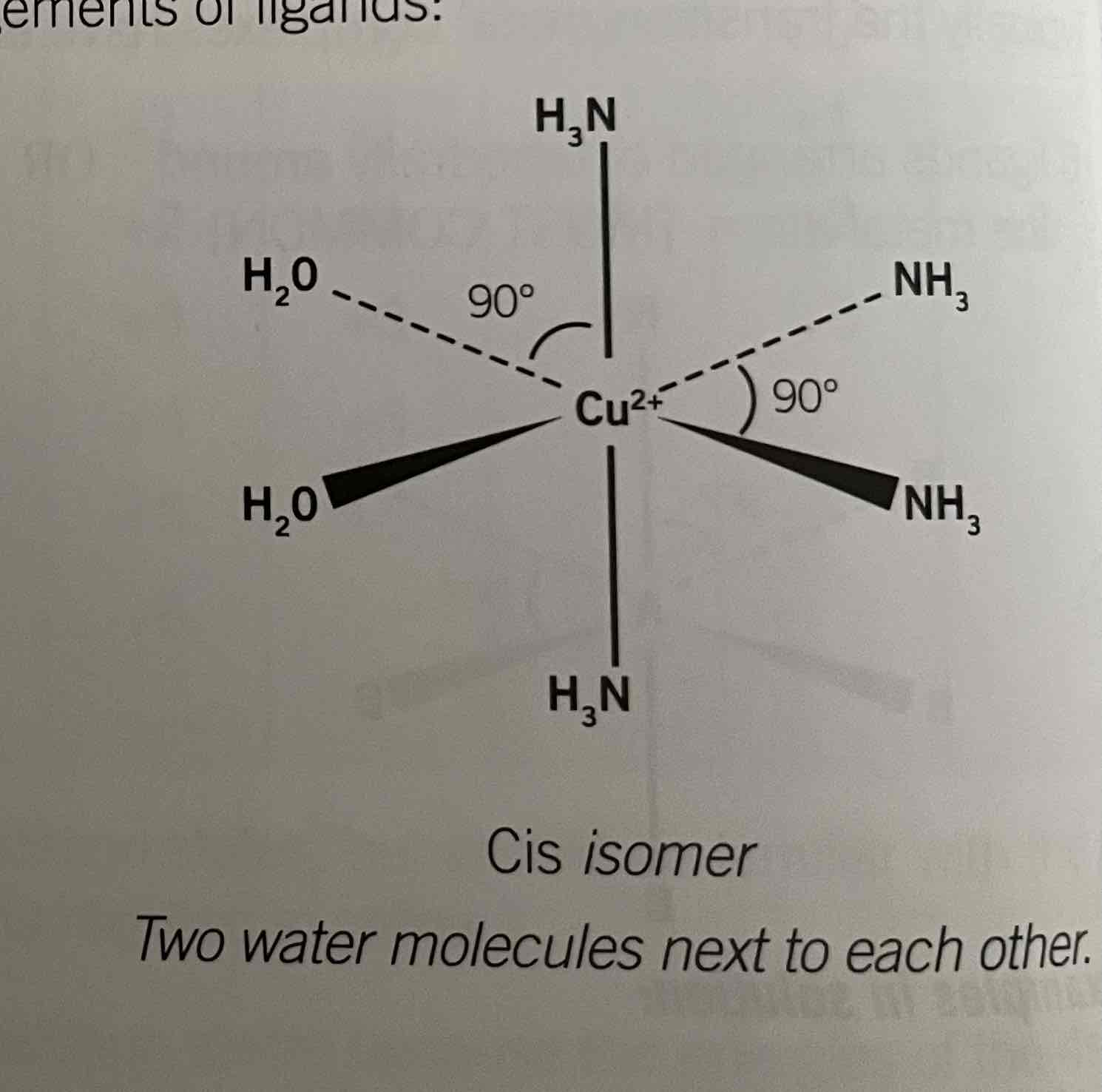
addition of ammonia causes for ammonia molecules to replace water. It's contains two different ligands.
Structure of [CuCl4]2- and [CoCl4]2-
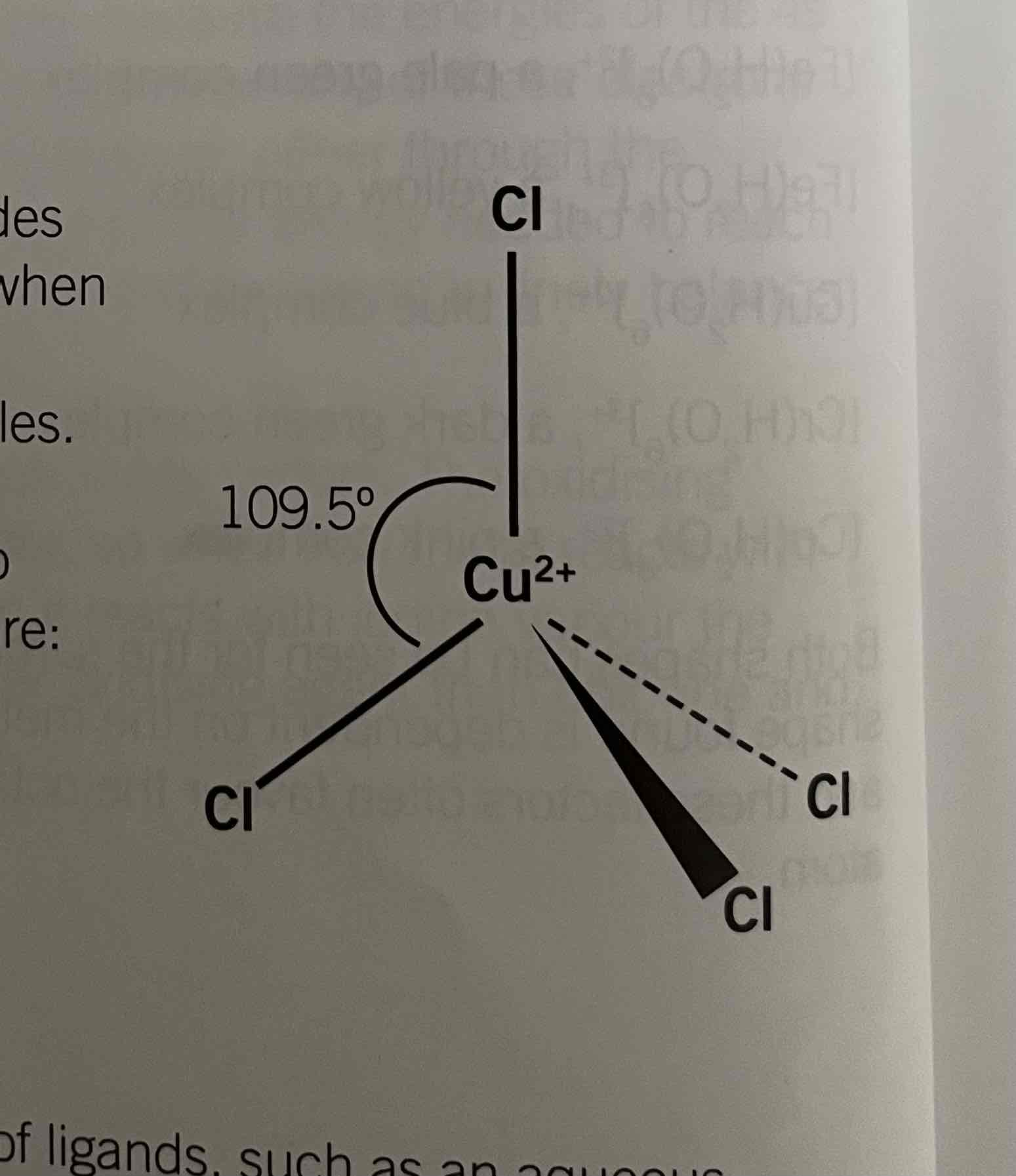
complexes are formed when copper or cobalt ions react with concentrated hydrochloric acid which displaces the water molecules

Ligand exchange
when a transistion
exchanged
When a transition metal ion is exposed to a mixture of ligands they can be exchanged to form a new complex, equilibrium process.
Ligand exchange with [Cu(H2O)6]2+
NH3
[Cu(H2O)6]2+ + 4NH3 = [Cu(NH3)4(H2O)2]2+ +4H2O
equilibrium to the right producing more [Cu(NH3)4(H2O)2]2+
addition of extra water forces the equilibrium to the left producing more [Cu(H2O)6]2+ complex.
Ligand exchange with [Co(H2O)6]2+
Cl
[Co(H2O)6]2+ + 4Cl- = [CoCl4]2- + 6H2O
if large amounts of chloride is used eg. by adding concentrated hydrochloric acid the equilibrium shifts to the right