MEDI1000: Microbial Control
1/55
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
Cleaning
Removal of visible soils, e.g. food residue, dust, dirt, corrosion, scale, grease
Microorganisms are removed but not killed
Often achieved with water and detergent
Sanitation
Destroys various microorganisms → reducing numbers to meet public quality + health standards
Usually not effective in presence of organic residues and detergents
Moist heat (steam), or chemicals (chlorine)
Disinfection
Removal of pathogens only
Killing them, or rendering them inert
Sterilisation
Removal of all microbes including bacterial spores
Germacide
An agent that kills pathogenic microorganisms
Disinfectant
Substance that removes or causes of destruction of harmful microbes (not usually spores) from inanimate objects
Antiseptic
Disinfectant for animate objects
Heat (pasteurisation)
60-80 degrees for a few minutes
Kill pathogens, destroy most other bacteria that cause food spoilage
E.g. preservation
Developed by Louis Pasteur mid 1800s - prevent spoilage of wine
Low temp, long time (LTLT) - 63 degrees, 30min
High temp, short time (HTST) - 72 degrees, 15 sec
Reduce spoilage bacteria
Extends shelf life
Radiation
UV rays (non-ionisation)
Infrared radiation (non-ionising)
Ionising radiation (energy to liberate an electron from an atom)
UV
UV rays (non-ionisation)
Bactericidal wavelength 200-295nm
Damages proteins and nucleic acid
Low penetrating power
Moderate exposure time
Reduction of microbes including air, water + surfaces
Ionising
Most effective: electron beam
Gamma rays (need lead sheilding)
X rays
Damage DNA by disrupting chemical bonds in cells
Prolonged exposure = sterilisation can be achieved
Methods of Disinfection
Heat (pasteurisation)
Radiation
Heat (boiling)
Chemical methods (solutions)
Modes of action for disinfection
Protein coagulation/denaturation
Disruption of cell membrane
Chemical antagonism (inactivation of enzymes)
Examples of Chemical Disinfectants
Alcohols
Aldehydes
Halogens (iodine)
Heavy metals & their components
Phenols and phenolic compounds
Examples of disruption of cell membrane for disinfectants
Surface active agents
Halogens (chlorine)
Chorhexidine
Phenols
Examples of chemical antagonism for disinfectants
(Inactivation of enzymes)
Metals
Organic matter modes of inhibition
Forms a precipitate with disinfectant - removes disinfectant from contact w bacteria
Reacts with the disinfectant to produce a non-bacterial agent(s)
Coats bacteria - protects bacteria from the disinfectant
Alcohols
>60% (70% optimal: osmosis, water and alcohol moves into cell)
Ethanol and isopropanol
Akin antiseptic and surface disinfection of objects
Disadvantages: lengthy exposure, low penetrating power, inactivated by organic substances, low activity against spores and non-enveloped viruses
Aldehydes
Formaldehyde & gluteraldehyde
For objects only
Non corrosive to metals
Disadvantages: tissue fixatives, strict safety precautions used when handling
Iodine
Halogens
Effective against bacteria, fungi, endospores, many viruses
Iodine tincture, povidone iodine
Povidone-iodine
Betadine
Antiseptic for small wounds and infections
Pre-op, skin disinfection
Good residual effect
Effective on a wide range of microbes
Disadvantages: skin discolouration, hypersensitivity, pseudomonas able to grow
Metals
Mercuric chloride
Copper salts
1% silver nitrate
Phenols
High conc. = denature proteins
Low conc. = damage cell membranes - inactivated by organic material
Corrosive and toxic (disinfectant use only)
Phenolic compounds
Skin antiseptic
Hexachlorphene
Soap, lotion, cosmetic products scrub
PHISOHEXXXXX
Trisoclan
Chloroxlylenol
Surface active agents (surfactants)
Quaternary Ammonium Compounds (QUATS)
Usually regarded as low level disinfectants
Inactivated by soaps
Pseudomonas can grow in QUATS
Some are used as antiseptics in mouth washes, lozenges, eye drops
Chlorine
Sodium hypochlorite (disinfectant)
Cheap bleach - 1% for body fluid, spills, 0.1% for contaminated equipment
Broad spectrum
Corrosive at high conc.
Long exposure time to be effective, inactivated by organic material
Chlorhexidine
Skin antiseptic
Oral mouth wash
Anti-bacterial, fungal, viral
Guidelines for disinfectant use
Use more reliable sterilising methods - don’t overuse chemicals
Clean before disinfection (remove organic matter)
Ensure total surface contact
Use recommended concentration
Correct exposure time
Fine after chemical disinfection
Antiseptics and ointments - avoid contamination
Reasons for disinfectant failure
Wrong conc.
Container not clean old solution topped up with new
Solution too old
Inactivation by chemicals, organic matter, etc
Wrong temp or pH
Inefficient exposure time
Object not completely immersed
Asepsis in clinical practice
Hand washing: with running water + drying removed most transient material, alcohol-based sanitisers, soap, chlorhexidine with water
Personal cleanliness: masks, gloves, protective clothing
Disinfection/sterilisation of work surfaces and equipment
Use of aseptic techniques
When you should sanitise/wash your hands (5)
Before touching patient
Before a procedure
After a procedure/body fluid exposure risk
After touching a patient
After touching a patient’s surroundings
Most to least effective controls
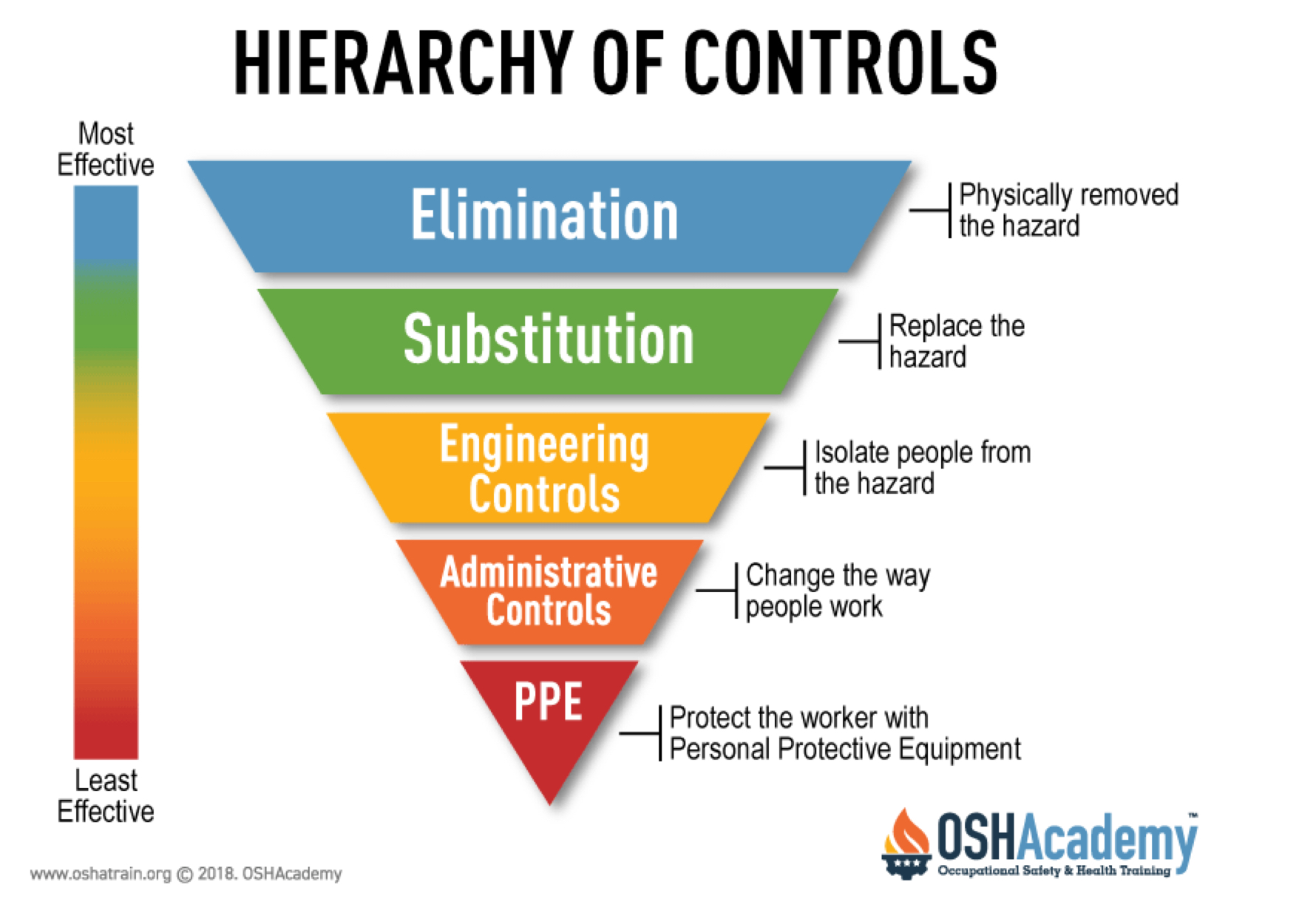
Why do we use face masks?
Protects others from mouth and nasal droplets
Large droplets w most particles + bacteria trapped - small droplets droplets get thru
Reduced projectiles spread of small droplets (travel less)
Reduced inoculum for people in proximity (doesn’t completely stop ALL droplets
Effective in reducing infection, especially with social distancing
Sneezing, coughing and scratching under mask will reduce effectiveness
Sterilisation
Process by which all microbes, including endospores are destroyed
When/where is sterilisation required?
Medical implements used on/in patients/labs - dressings & surgical implements, catheters + prosthetic devices, solutions (saline, antibiotics)
Culture media, glassware
Medical waste
For canned and frozen meals
Most often achieved by moist heat under pressure (autoclaving)
Bacterial spores
Most heat resistant and difficult to kill microbial structure
Thick spore coat protects from radiation + chemicals
If all spores destroyed → so are all pathogens (except prions)
Conditions for moist heat sterilisation are based on killing bacterial spores
Prions
Proteinaceous infectious particles
Difficult to destroy by standard methods (resistant to ionising radiation, resistant to formaldehyde
CNS high tissue risk
Decontamination procedures for non-disposable, heat sensitive instruments
Sterilisation by Moist Heat
Moist heat (autoclave) more effective than dry heat (hot air oven)
Moisture = better conductor of heat and better heat penetration
Mode of action - coagulation & denaturing of proteins
Temp achieved & exposure time important → 15min @ 121 degrees at 15psi above ambient atmospheric (most common)
Precautions: loose caking of items to allow steam penetration, loose lids on bottles
Sterilisation indicators
Autoclave printouts/monitoring
Biological - spore strips: endospores impregnated on paper strips inside porous envelope and placed inside autoclave, transfer to broth, incubate 55 degrees for 5 days → growth = failed, no growth = efficient yay
Autoclave tape (Bowie-Dick test): stripes on tape are white, pack with lines still visible = properly sterilised, immediate result, indicates 121 degrees but doesn’t indicate time held
Hot air sterilisation
2hrs at 160 degrees or 1hr at 10degrees
Advantage: glassware, oils, powders, doesn’t blunt shear objects
Disadvantage: less efficient, dried proteins resist denaturation
Incineration sterilisation
Bunsen burner and incinerators
1 second @ >1000 degrees
Microbiological loops, Medical waste, Animal carcasses
Sterilisation by gases and cold sterilisation
Mode of action - denaturation of proteins
Good for heat sensitive medical plastics, optical instruments
Whole rooms (e.g. cold rooms) & mechanical apparatus
E.g. formaldehyde, glutaraldehyde, ethylene oxide, plasma sterilisation
Sterilisation by filtration: fluids and air
Membrane filtration of fluids
Filter bacteria out 0.45um or less
Applies to good quality, under sink water filters
Doesn’t apply to normal filters (eg. Jug, cartridge)
Filter out bacteria and virus particles (pores of 0.01um or 10nm)
High efficiency particulate air (HEPA)
Pre filter: removes large particles, room odour
Capable of removing 0.2 micrometer particles → 99.97% accurate
VOC filter - removes volatile organic compounds & chemical substances
Some have randomly arranged fibres, pre-filters are used to remove large particles that old rapidly clog a HEPA filter,
Pore sizes usually larger than 0.3um → function relies on diffusion, interception and impaction
Antibiotics
Natural compounds produced by microorganism that kill or inhibit other microorganisms
Can be synthetic or semi-synthetic
Synergism
Compounds that act together to enhance/improve effect
Antagonism
Compounds that act against each other to reduce effect
Bacteriostatic
Compounds that inhibit growth of microorganism (bugs that still viable)
Bactericidal
Compounds that kills microorganisms
Antibiotics - The Beta-Lactam Group
One of the largest groups of antibiotics
Inhibit the last stage of the cell wall production process
Kill bacteria without damaging cells of the host (eukaryotes don’t have a cell wall)
However, Bacteria express beta-lactamase enzymes - some resistance, multiple different resistance genes are possible
The Beta-Lactam ring structure
Common to all beta-lactam agents
Ring must be intact for antibacterial activity - inactivated by beta-lactamase enzymes of the bacteria
Binds to proteins @ cell membrane interface involved in cross-linking of peptidoglycan stands → cell lysis
All beta-lactams are Bactericidal
Not effective against resisting bacteria
Antibiotic resistance
Over prescription = over exposure = selective pressure
Not o pelting a course of antibiotics (that work) = electing resistant mutants
Use in intensive farming of animals - growth promoters → resistant animal strains of bacteria
Completely resistant and untreatable tuberculosis strains
Antibiotic Creed

Problems with broad-spectrum antibiotics
Prolonged use = greatly reduced NF at some body sites → increase risk of second infection
Genital tract and oral cavity → yeast not affective against antibiotics
GIT → gastrointestinal infections
Anti-fungal treatment
Antibacterial antimicrobials ineffective against fungi
Difficult to treat because eukaryotic
Treatment usually long term + toxic!
Examples of antifungal drugs: Flucytosine, fluconazole, itraconazole
Antiviral chemotherapy
Drugs that bind free virus preventing entry
Prevention of uncoating of virus
Inhibit viral replication
Interfere with viral release