Quantitive treatment of substances and chemical formula
1/7
Earn XP
Description and Tags
Important vocab, practice formulas :)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
Atomic weight (average atomic mass)
= Sum of isotope masses multiplied by their abundance / abundance of all isotopes (usually 100)
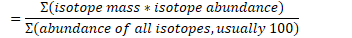
Molecular or formula weight
A compound’s or molecule’s sum of the atomic weights of all the individual atoms in the molecule
Molar mass
Element’s atomic mass in grams per moles
Amount of substance
Measures in moles. One mole is defined as the Avogadro’s number’s amount of particles.
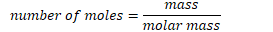
Molar concentration (molarity)
number of moles of solute per liter of solution (mol/L)
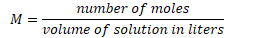
Expressed: [Na+] "the molar concentration of sodium ions"
Mass percent concentration (weight percent)
ratio of the mass of the solute to the total mass of the solution in %
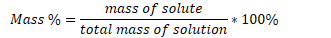
The ideal gas law equation
pV=nRT
R = ideal gas constant