polyatomic ions and lab tools
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
hydroxide
OH-
nitrate
NO3-
Sulfate
SO4-2
carbonate
CO3-2
phosphate
PO4-3
chromate
CrO42-
chlorate
ClO3-
ammonia
NH4+

watch glass

evaporating dish
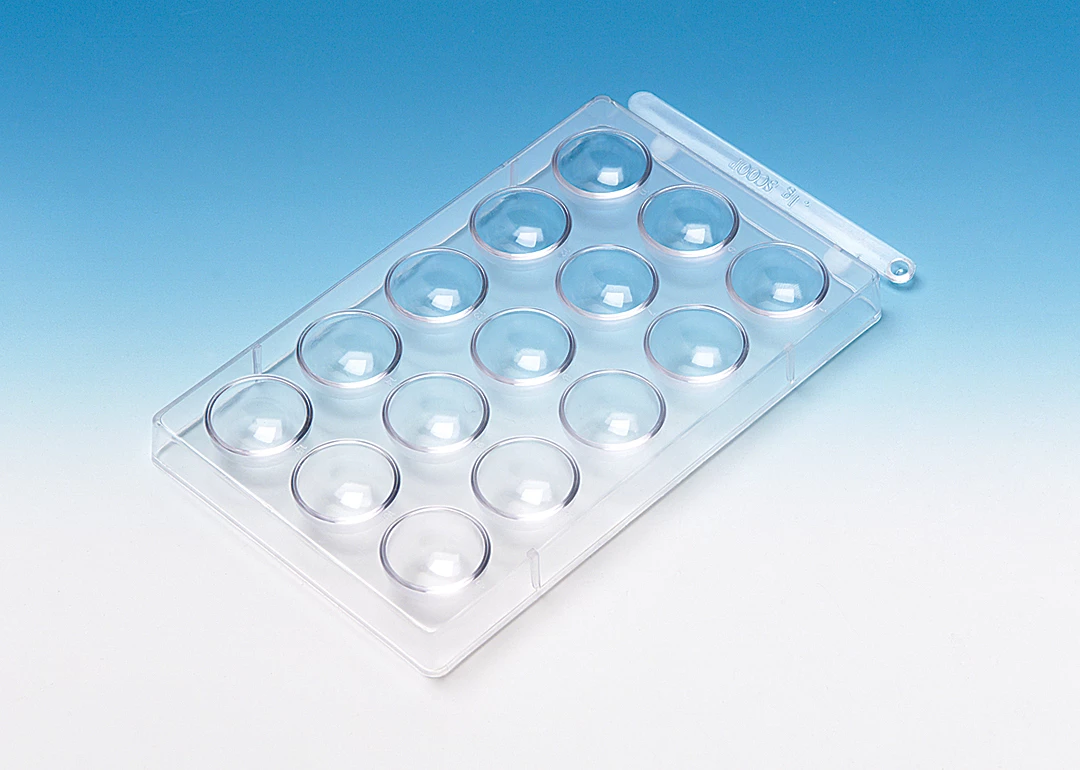
spot well plate

hot plate

ring stand

ring

stirring rod

spatula

erlenmeyer flask

electronic balance

buchner funnel
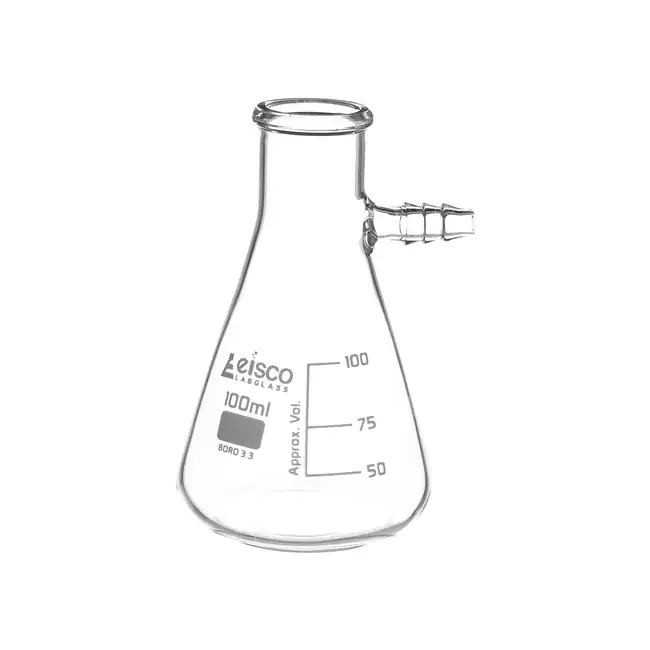
sidearm flask

wire gauze

striker

dropper
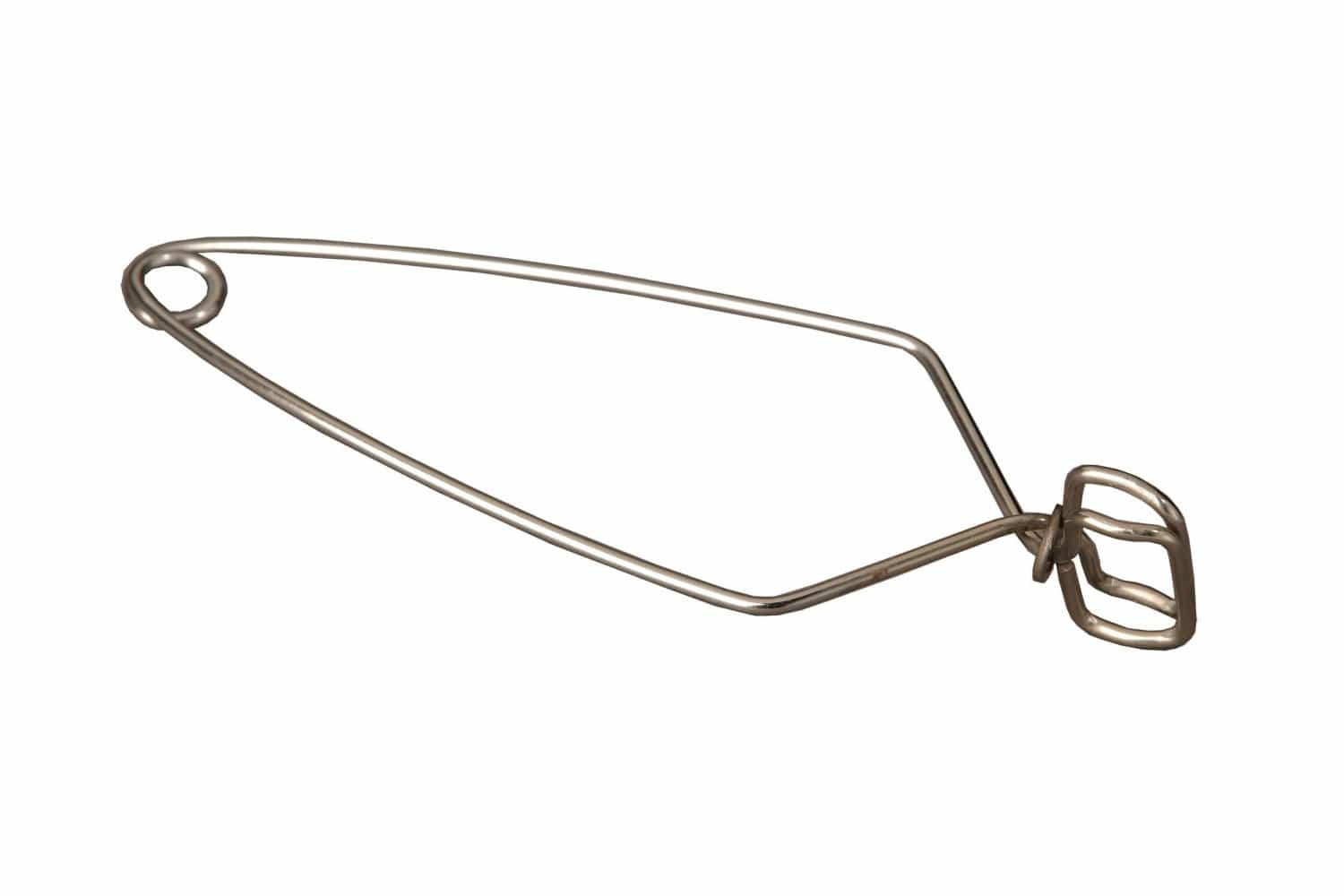
test tube holder

test tube rack
mass to mass
g x amu/1 M x M/M x amu/1 M
mass to moles
g x 1 M/amu x M/M
moles to mass
M x M/M x amu/1 M
moles to moles
M x M/M
molarity
moles of solvent/liters of solution
mass percent
grams of solute/grams of solution x 100
change in state
temp increase
change in size
gas bubble formation
tearing apart
permanent color change
breaking into pieces
formation of precipitate
grinding into powder
odor change
dissolving
change in pH