IGCSE Chemistry
1/140
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
141 Terms
Examples of mixtures
water and iron filings, salt and sand, pepper and water
3 groups of elements
metals, metalloids, non-metals
Properties of metals
good conductors of heat and electricity, malleable, ductile, shiny, solid at room temperature
Examples of metals
Gold, copper, aluminum
Properties of non-metals
poor conductors of heat and electricity, not ductile or malleable, dull, brittle, mostly gases
Examples of non-metals
oxygen, carbon, chlorine
Atom
The smallest particle of an element
Element
A pure substance made of only one kind of atom and cannot be broken down into simpler substances
Examples of elements
Hydrogen, Helium, Lithium
Compound
A substance made up of atoms of two or more different elements chemically combined together and can be separated by chemical means
Examples of compounds
water, carbon dioxide, sucrose
Mixture
A combination of two or more substances that are not chemically combined and can be separated by physical means
Properties of metalloids
They have properties of both metals and nonmetals
Examples of metalloids
germanium, silicon, arsenic
Alloy
A mixture of two or more metals
Properties of alloys
They are usually stronger than metals
Why alloys are harder than pure metals
The regular layers in pure metal are distorted by atoms of different sizes in an alloy
Examples of alloys
Brass = Copper + Zinc
Bronze = Copper + Tin
Steel = Iron + Carbon
Transition metals
Elements that form a bridge between elements on the left and right sides of the periodic table
Properties of transition metals
Good conductors of heat and electricity
Examples of transition metals
gold, silver, copper
Nucleus
The dense, positively charged center of an atom made up of protons and neutrons
Particles of an atom
protons, neutrons, electrons
Mass of particles in an atom
electron--almost 0; proton--1; neutron--1
Charges of particles in an atom
electron--negative; proton--positive; neutron--neutral
Atomic number
The number of protons in an atom, also known as proton number
Mass number
The total number of protons and neutrons in the nucleus of an atom
Mass number formula
protons + neutrons
Relative atomic mass
A weighted average of the masses of the atoms of the isotopes and takes account of the abundance of each of the isotopes of the element
Relative atomic mass vs mass number
Mass numbers are always whole numbers while relative atomic masses are usually rounded off to the nearest whole number, but are not whole numbers
Number of neutrons formula
mass number - atomic number
Number of electrons
=number of protons
Isotope
An atom with the same number of protons and a different number of neutrons from other atoms of the same element.
Properties of isotopes
Same chemical properties, different physical properties (e.g. boiling/melting point)
Examples of isotopes
Carbon-12, Carbon-13, Carbon-14
Periodic table
A table that shows the elements, their atomic number, symbol, and average atomic mass; elements with similar chemical properties are grouped together.
Shell
An area in an atom, around its nucleus, where electrons are found
Period
A horizontal row of elements in the periodic table; it shows the number of shells an element has
Reactivity of metals along a period
reactivity decreases as you go from left to right across a period
Reactivity of non metals along a period
reactivity increases as you go from left to right across a period
Group
A vertical column of elements in the periodic table; it shows the number of valence electrons an element has
Reactivity of metals down a group
reactivity increases as you go down a group
Reactivity of non metals down a group
reactivity increases as you go up a group
Atomic structure
proton(in nucleus)
neutron(in nucleus)
electron(outside nucleus and in shells)
Ways to represent atomic structure
Electronic configuration, dot and cross diagrams
Electronic configuration
The arrangement of electrons in shells around the nucleus of an atom represented numerically
Example of electronic configuration
Oxygen - (2,6)
Sodium - (2,8,1)
Calcium - (2,8,8,2)
Isoelectronic
When two ions have the same electron configuration
Dot and cross diagram
A drawing to show the arrangement of the outer shell electrons only of the atoms or ions in a substance
Example of a dot-and-cross diagram
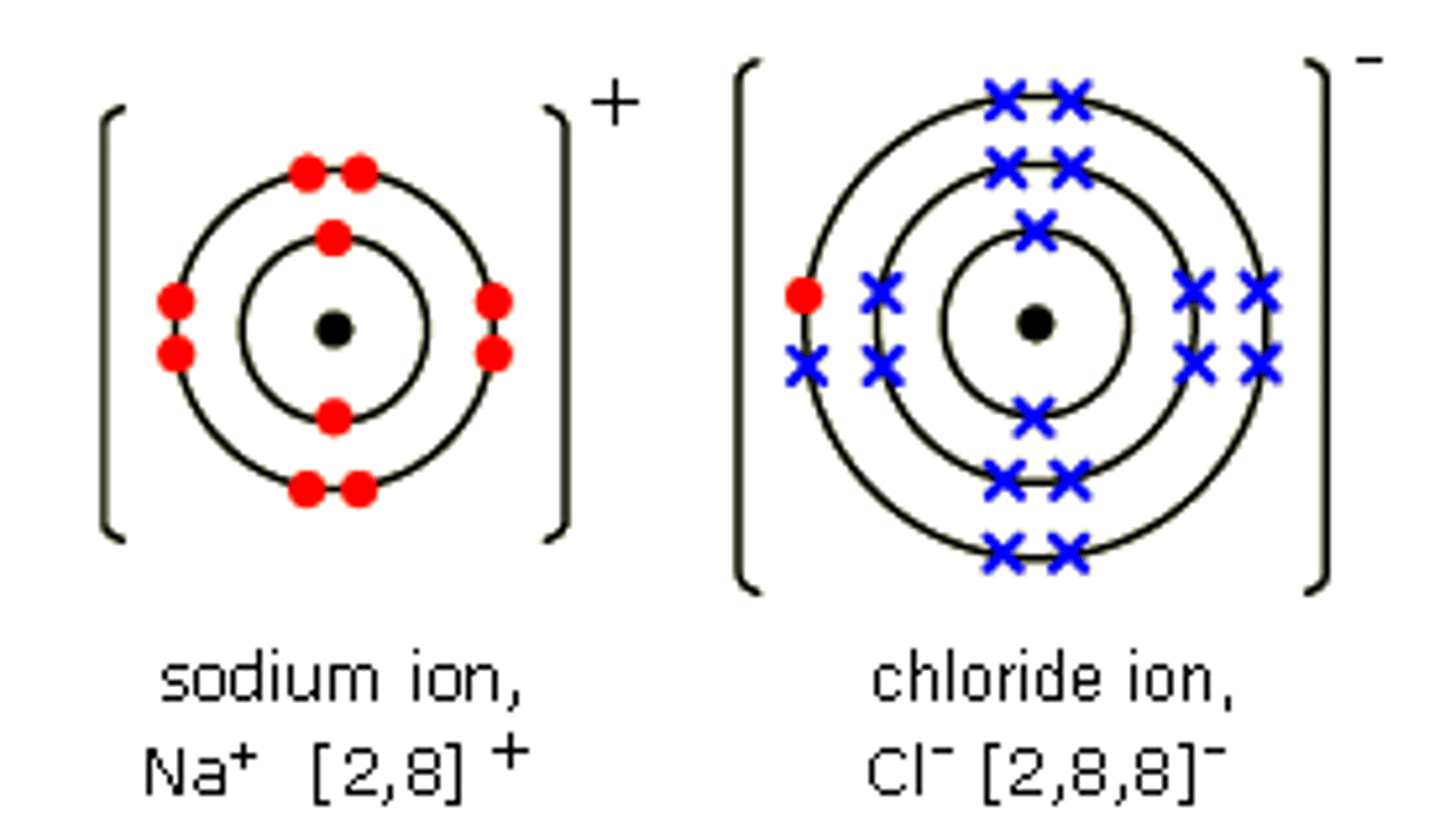
Maximum number of electrons in shells
1st shell: 2
2nd shell: 8
3rd shell: 8
Which shells are filled first?
1st shell, 2nd shell, 3rd shell and so on
Note: The previous shell should be completely filled first before drawing another shell
Chemical bonding
The joining of atoms to form new substances
Why elements bond
To become stable and have 8 electrons in their outermost shell
Elements that are stable
Elements in group 8 of the periodic table
Elements that are not stable
Elements from group 1-7 of the periodic table
How elements become stable
They have to either gain or lose electrons
Ion
An atom or group of atoms that has a positive or negative charge.
Charge
A measure of the extra positive or negative particles that an atom or group of atoms has
Cation
A positively charged ion or (has more protons than electrons)
Examples of cations
Sodium (Na+), Potassium (K+), Lithium (Li+)
Anion
a negatively charged ion (has more electrons than protons)
Examples of anions
Chloride (Cl-), Bicarbonate (HCO3-), Sulfate (SO4-)
Types of bonding
ionic, covalent, metallic
Ionic bonding
The electrostatic attraction between positive and negatively charged ions
Ionic bonding occurs between
metals and non-metals
Examples of ionic compounds
NaCl, MgO
Explain in terms of bonding and structure why ionic compounds have high melting points
Ionic compounds have high melting points because a large amount of energy is needed to break the strong ionic bonds in its giant ionic lattice
Why is NaCl not a conductor of electricity in the solid state but is a conductor of electricity in the liquid/aqueous state
NaCl is an ionic compound. In NaCl(aq) and NaCl(l), there are freely moving ions which can carry charges, therefore they are conductors of electricity. In NaCl(s), the ions are not free to move and are tightly packed in its giant ionic lattice. Therefore, they cannot carry charges and conduct electricity.
Covalent bonding
The electrostatic attraction between the positively charged nucleus and the shared pair of electrons
Covalent bonding occurs between
non metal and non metal
Examples of covalent compounds
CO2, H2O
Naming covalent compounds
Add prefixes( mono-1, di-2, tri-3, tetra-4, penta-5, hexa-6, hepta-7, octa-8, nona-9, deca-10) to elements and add -ide to the end of the anion
Explain why CO2 has a low melting and boiling point
The bonding in CO2 is covalent. A small amount of energy is needed to overcome the weak intermolecular forces of attraction in its simple molecular structure, hence it has a low melting and boiling point.
Giant covalent structure
A structure with many atoms joined to each other by lots of strong, covalent bonds giving a high melting point and (except for graphite) poor electrical conductivity.
Examples of giant covalent structures
Diamond, graphite, silicon dioxide
Explain why graphite has a high melting/boiling point
Graphite has a high melting/boiling point because each carbon atom is covalently bonded to 3 other carbon atoms, which in turn, are bonded to 3 more carbon atoms. This forms a continuous layer of hexagons. I is difficult to break these many strong covalent bonds, therefore it has a high melting/boiling point.
Why is graphite soft and slippery?
Graphite is soft and slippery because it is made up of man layers of carbon atoms on top of each other and they are held loosely by weak intermolecular forces of attraction between the layers so the layers can slide on top of each other when a force is applied, making graphite soft and slippery.
Why does graphite conduct electricity?
Graphite conducts electricity because each carbon atom has 1 outer electron that is not used to form covalent bonds. These electrons can move freely along the layers from 1 carbon atom to the next. They are said to be delocalized These free-moving electrons allow graphite to carry charges and conduct electricity.
Metallic bonding
The electrostatic attraction between the positively charged metal ions and the sea of delocalized electrons
Metallic bonding occurs between
metal and metal
Characteristics of metals
Good conductors, solid at room temperature (except mercury), luster, ductile and malleable
Explain in terms of bonding why metals have high melting/boiling points
Metals have high melting/boiling points because a high amount of energy is needed to break its strong metallic bonds in its giant ionic lattice
Chemical reaction
the process by which 1 or more substances change to produce 1 or more different substances
Endothermic reaction
A reaction in which energy is absorbed
Exothermic reaction
A reaction that releases energy in the form of heat
Chemical word equation
uses words to describe the reactants and products(reactant + reactant -> products)
Example of chemical word equation
oxygen + hydrogen -> water
Balanced symbol equation
An equation written in such a way that the number of each type of element is equal on both sides.
Example of balanced symbol equation
2H2 + O2 -> 2H2O
Steps to write a balanced equation
Step 1: Make sure you know what the reactants are
Step 2: Write out the word equation
Step 3: Write out the equation using the formulae of the elements and compounds
Step 4: Balance the equation
Types of chemical reactions
synthesis, decomposition, single replacement, double replacement, combustion
Synthesis
a chemical reaction in which two or more substances combine to form a new compound (A + B --> AB)
Decomposition
a chemical reaction that breaks down compounds into simpler products (AB --> A + B)
Single replacement
a chemical reaction where one element replaces another element in a compound (A + BC --> AC + B)
Double replacement
a chemical reaction where two elements in different compounds trade places (AB + CD --> AD + CB)
Combustion
a chemical reaction between oxygen and fuel that results in carbon dioxide and oxygen
State symbols
They show the state of a substance
(s) solid
(l) liquid
(g) gas
(aq) aqueous
Aqeuous
dissolved in water
Solubility rules
1. Na and K salts are soluble
2. NH4+ salts are soluble
3. NO3- is soluble
4. Ethanoates are soluble
5. So4 2- soluble except CaSO4, BaSO4, PbSO4
6. Cl- soluble except AgCl and PbCl2
7. CO3 2- insoluble except NaCO3, K2CO3, and (NH4)2 CO3