chem1aa3 organic chem
1/59
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
Nucleophile
Nucleus loving electron donor Lewis base
Électrophile
Electron loving, electron acceptor Lewis acid
DO E isomers of aliénés have higher melting points than Z-isomers.
YES
Electron flow and curved arrows
Curved arrows used to show how electrons move in bond forming and breaking processes
Rules:
Represents movement of pair of electrons
Start from source of electron density to source of electron deficiency (-ve to positive)
When forming a bond, arrow starts from source of electrons and moves towards an atom
When breaking a bond arrow starts from the center of a bond and moves towards atom accepting electrons
Organic reactions key concepts
nucleophile provides a source of electron dentistry often contains a lone pair of electrons or electron density in a pi bond
Electrophole has a lack of electron density, often a carbon atom bonded to an electronegative atom. Called a leaving group
Curved arrows are drawn with the end of the arrow at the nucleophile and head pointing at the électrophone
When methyl or primary alkyl haloes are used
Rate law is kobsAB
methyl alkyl halodes have a large k than primary alkyl halides
Nucleophile is involved in rds
When tertiary alkyl halides are used
Rate law is jobs=A nucleophile is not involved in rds
Secondary alkyl halides are used
Condition dependant experience both rate laws
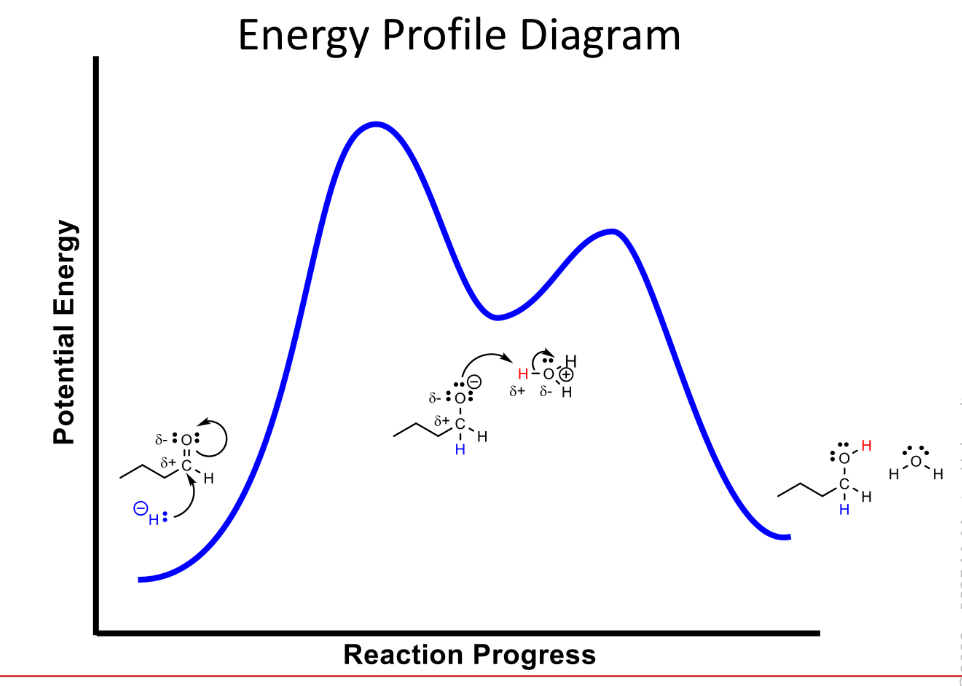
Nucleophoillic substitution mechanisms
Addtive
Concerted
Dissociative
Additive
from bond then break bond a molecule adds across a multiple bond of another molecule
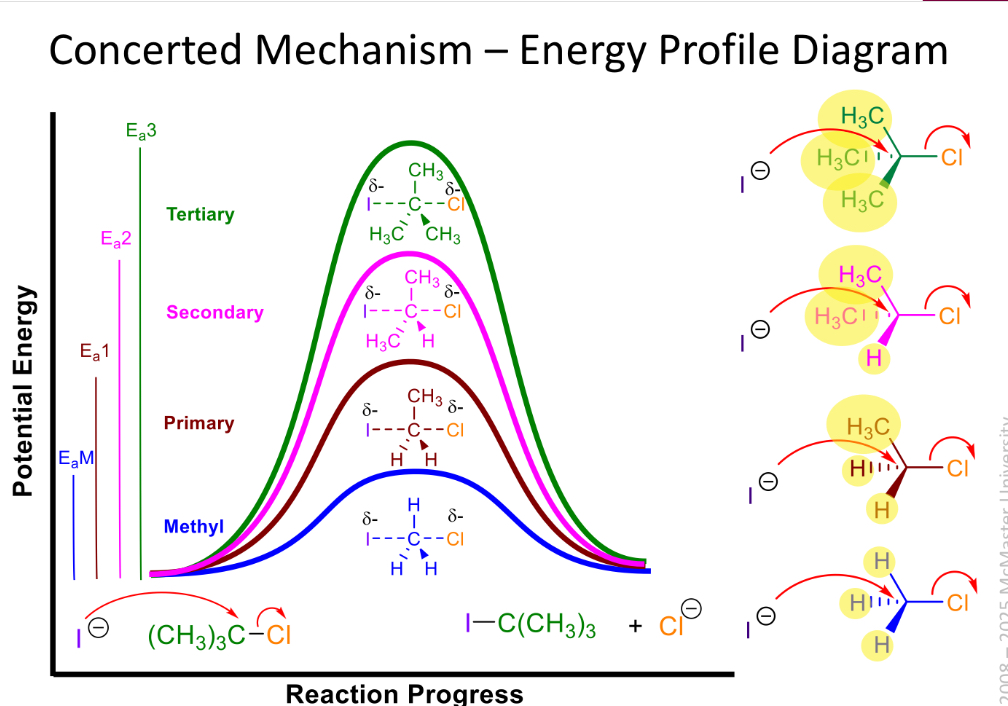
Concerted draw energy profile diagram
Bonds breaks and form simultaneously
activation energy increases as electron density around central carbon decreases
Rate=k(alkyl halide)(nucleophile)
2nd order overall
1 step mechanism
Sn2
nucleophillic substitution alcohols, ethers, esters
hydroxides, alkoxudes, and carboxylates can be used as nucleophiles to make alcohols, ethers, esters, respectively
Rate is k(alkyl halides)(nucleophile)
Water or alcohol can be used as nucleophile and solvent to generate alcohol or ether w a tertiary alkyl halides via sn1
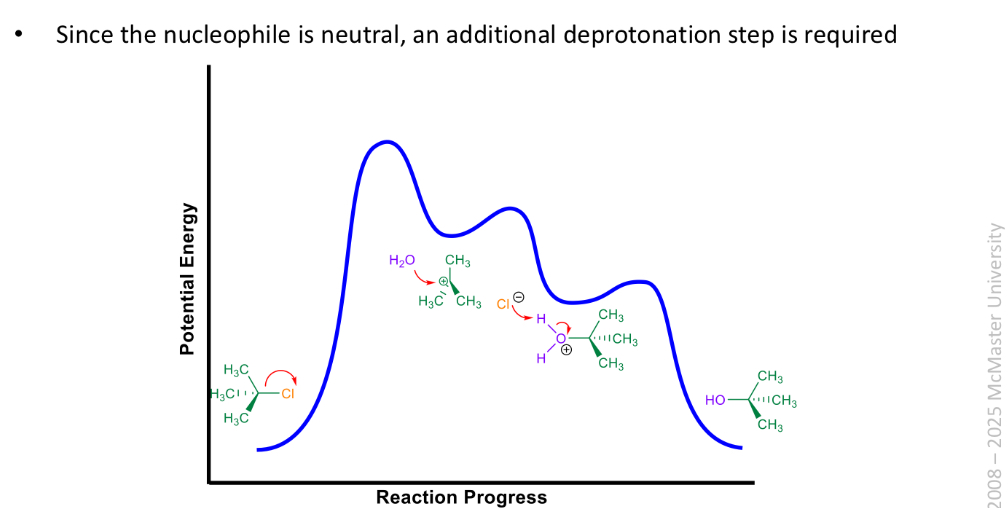
Sn1 vs Sn2
mechanism depends on many factors
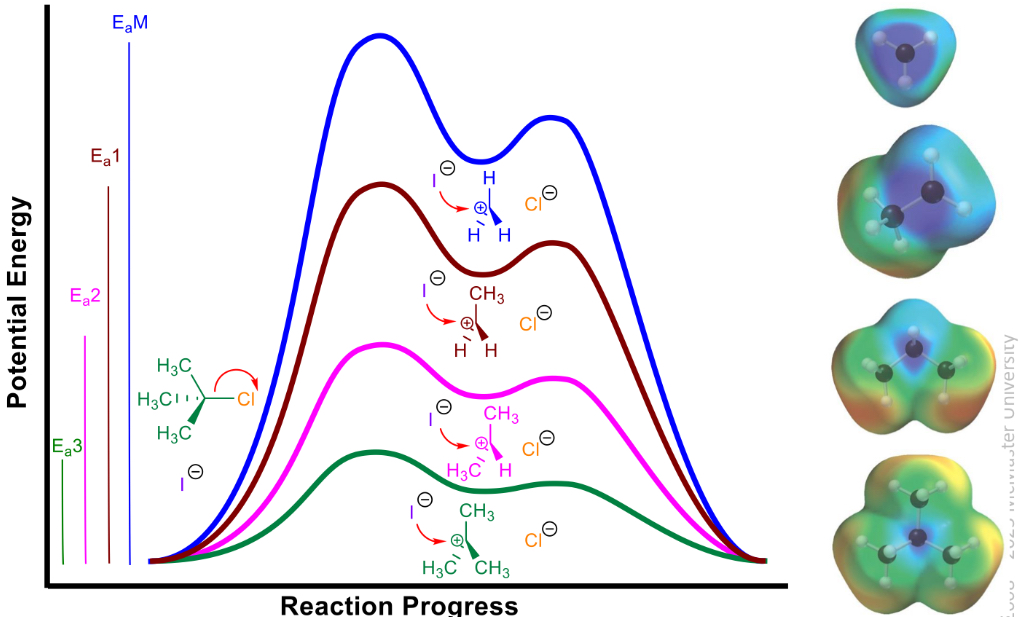
1 degree electrophile = sn2, less stable carbocation intermediate, less steric hindrance to nucleophile sttack
2 degree electrophole hard to predict generally stronger nucleophiles ones favour sn2
Third defree electrophile is sn1, since more stable carbocation intermediate, more steric hindrance to nucleophillic sttack
Dissociative and draw energy diagram
Breaks bond then form bond
activation energy decreases as electron density around central carbon increases
Rate is k(alkyl halide)
1sr order
2 step
Sn1
Hydrohalogenation of alkenes
When HX adds to the double bond of an alkene
The h atom adds to the carbon with the smallest number of alkyl groups
The X atom adds to the carbon atom with the largest number of alkyl groups
Called markovnikovs rule
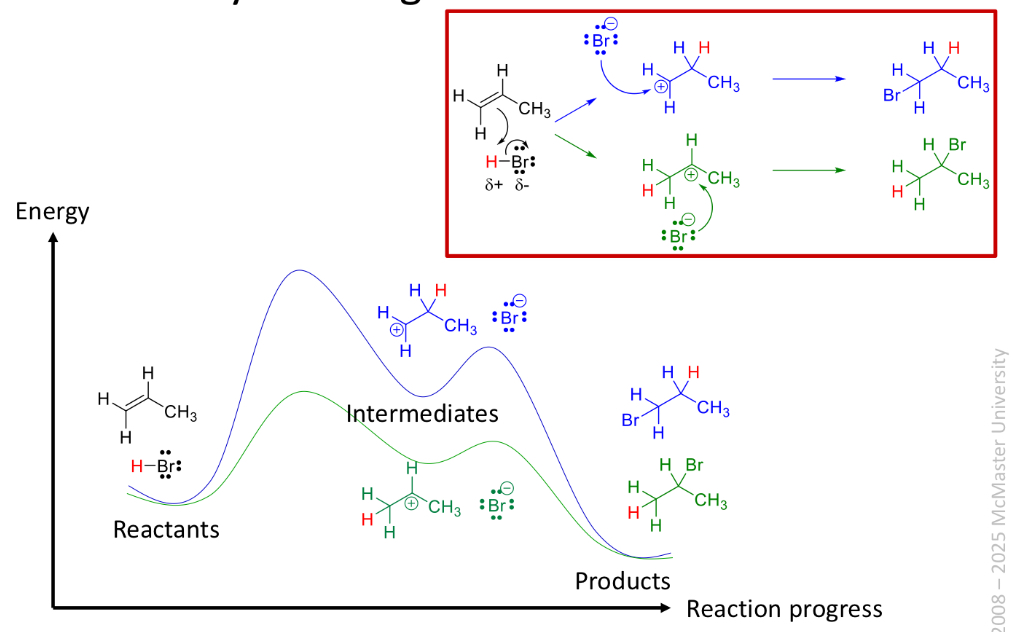
Roles in addition reaction
Step 1: alkene is nucleophile and HBr is the electrophile
Step 2: bromide = nucleophile carbocation intermediate = electrophile
Relative stability of carbocations
Stability increases with number of alkyl substituents
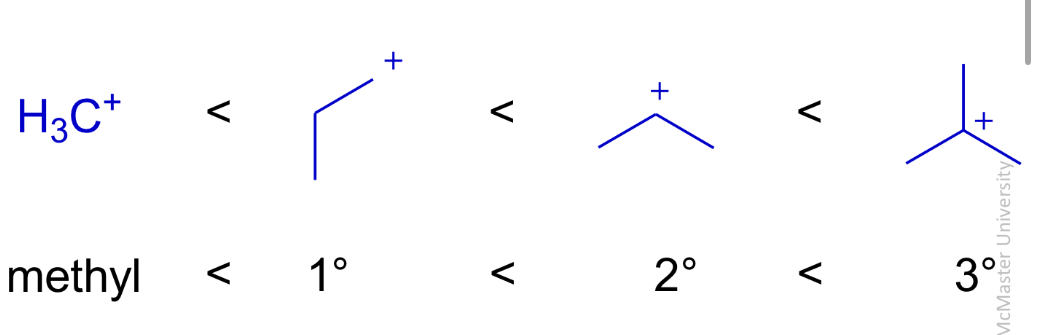
Hydration of alkenes
Alkene plus water turns into alcohol
Forward reaction favoured in dilute h2s04 (10%)
Its conjugate base won’t add to cation and Hcl would lead to mixture of hydration and addition products
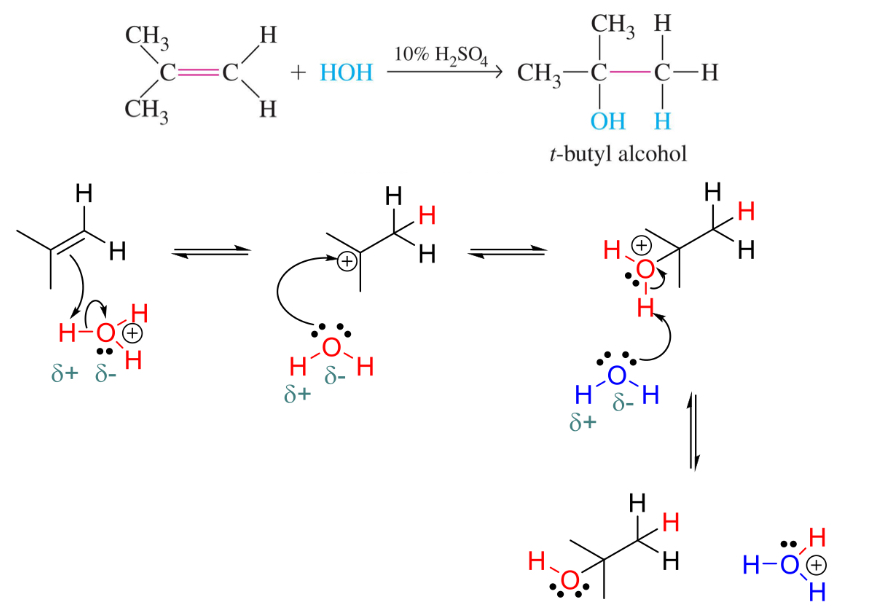
Hydrogénation (réduction) of alkenes
alkene plus h2 and catalyst
Catalyst usually grp 10 metal (Pd, Pt, Ni)
SYN addition (h atoms add to same face if alkene)
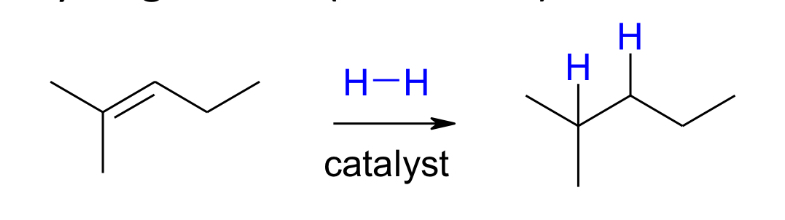
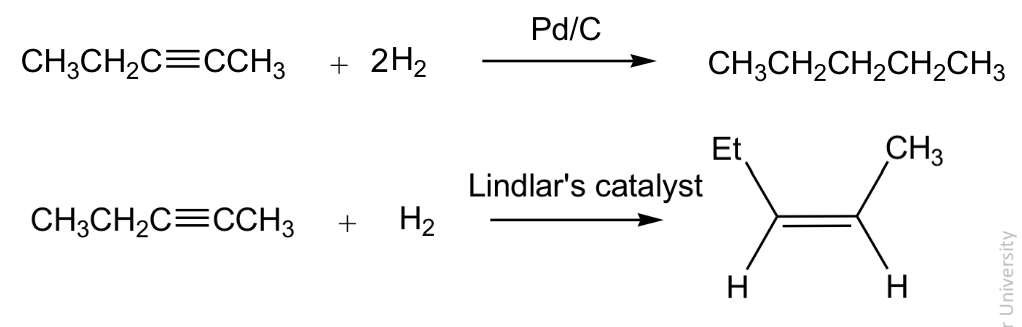
Hydrogénation (réduction) of alkenes
similar to hydrogénation of alkenes
Lindlars catalyst is deactivated (poisoned)
Syn addition results in only the z or cis isomer
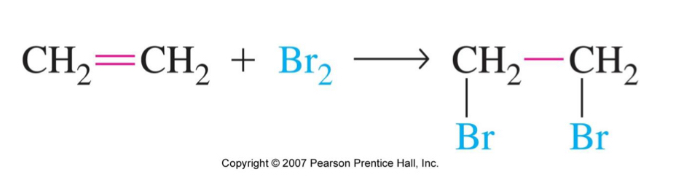
Halogénation of alkenes
bromination adds one Br to each alkene carbon c
The result is dibromoalkane
Classifying alcohols
primary when attached to carbon attached to one another carbon
Secondary when attached to carbon attached to two other carbons
Tertiary when attached to carbon attached to three other carbons
Oxidation
Gain an oxygen or lose two hydrogens
Réduction
Lose an oxygen or gain two hydrogens
Primary alcohol oxidation
Aldéhyde
Carboxylic acid
Requires oxidizing agent
Secondary alcohols oxidation
requires oxidizing agent
Turns into ketone
Tertiary alcohol oxidation
a Carbon bond would have to break for oxidation to occur
Therefore, no reaction
Oxidizing agents
usually metals in high oxidation states (transfer 2 to 4 electrons)
I.e mno4, cr2o7 2-,
Usually done in acid or base to facilitate electron transfer
Pyridinum chlorochromste (PCC in CH2Cl2) is specific to oxidizing secondary alcohol to ketone does one step only
Reduction of carbon double bond vs carbon double bonded to oxygen group
*Fifure Otto Stefan
Structure of sodium borohydride nabh4
source of nucleophillic hydrogen that can be used in carbonyl reduction reactions
Since hydrogen is more electronegative than boron, it holds most of the negative charge on nabh4
Sodium behaves as a counter ion spectator in borohydride reduction reactions
Désignation of saturated carbon center as 1,2,3,4
Based on how many other carbon atoms it is bonded to
Valence bind theory
electrons reside in quantum mechanical orbitals localized on individual atoms
Orbitals could be atomic SPDF or hybridized ie sp3 sp2 sp
A chemical bond results from the overlap of two half filled orbitals in which the electrons have opposite spins
The shape of the molecule is determined by the geometry of the overlapping orbitals
Hybridization
mix two or more standard atomic orbitals to form new hybrid orbitals
I.e sp orbitals
Minimizes energy of molecule by maximizing orbital overlap in a bond
VSEPR vs hybrid orbitals
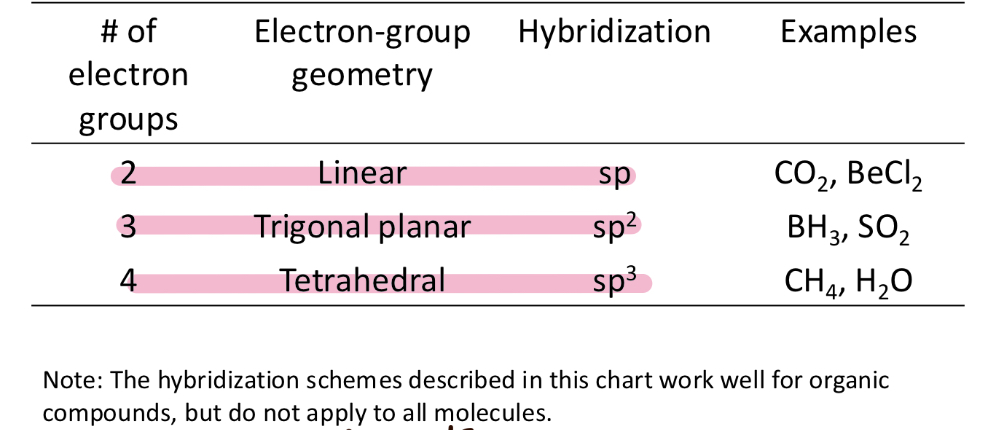
Rules of hybridization
The number of standard atomic orbitals mixed together is equal to the number of hybrid orbitals formed
The combination of standard atomic orbitals mixed together determines the shapes and energies of the hybrid orbitals formed
Hybrid orbitals
hybrid means something of mixed origin or composition
Hybrid orbitals arise by combo of atomic orbitals
Upon hybridization
Total number of orbitals is conserved
Orbital energy is conserved
The average energy of the electrons in hybrid orbitals is higheR compared to electrons in atomic orbitals
Hybridization results in slightly higher potential energy thus would not happen at zero kelvin
Counting number of sigma bonds
count each single bond
Count each double bond
Count each triple bond
Counting pi binds
count number of double bonds
Count triple bonds and multiply by 2
Sigma bond
orbitals overlap end on
Electron density along inter nuclear axis
Only one sigma bind possible between two atoms
Generally stronger and harder to break
Pi bond
Orbitals overlap side on
Nodal plane along internuclear axis
Up to two pi bonds possible between two atoms
Generally weaker and easier to break
Single bond vs double bond
single bond can have different conformations and the atom arrangement can be changed by rotation about single without breaking any bonds
Double bonds have different configurations resulting from, spatial arrangement of bonds
Changing atom arrangement requires breaking the pi bond
Restricted rotation
Chemistry if vision
rods and cones contain proteins bound to 11-cis-retinal
Light stiles the molecule breaking the pi bond
Sigma bond stays intact allowing for rotation
Different shape of trans retinal causes changes in the bound protein. And a signal is transmitted to the brain
Melting point of fatty acids
linear chain close packing with many IMF means highest melting point
Kinks from tnrans double bonds reduce packing efficiency lower melting point
Cis double bonds interfere with packing and IMF meaning lowest melting point
E/Z nomenclature
E higher priority groups ch3 are on opposite sides of the double bond
Z higher priority groups ch3 are on the same side of the double bond
For molecules w different groups attached to carbon double bond e/z used instead of cis/trans
Can-Ingold-Prelog rules
use atomic number to define priority for each pair of atoms bound to each carbon in the carbon double bond
F9 higher than n7
Relationship between two high priority groups defines the configuration.
Opposite sides means e
Same sides means Z as in together
When groups are different but have identical atoms bound to c double c, move on to each atom connected to that atom until a point of difference is found
Brainwashing bees
QMP queen mandibular pheromone causes young workers to feed and groom her
Suppresses new queens controls colony behaviour
Contains homovanillyl alcohol or HVA suppresses bad memories but not good ones
Hypothesis: HVA mitigates unpleasant side effects of QMP
HVA Lowers dopamine associated with learning
HVA molecules can treat high dopamine psychoses and schizophrenia
But needs high specificity (ADHD and Parkinson’s linked to low dopamine) could lead to undesired side effects
When will a molecule have no geometric isomers
if two substituents on a given carbon atom are identical. 2-methyl-2-butene does not have any E- or Z-isomers, because the substituents on carbon #2 are identical (both methyl groups):
Statement “In alkenes the unhybridized p-orbitals form a π-bond and force the substituents attached to the alkene C atoms to become eclipsed.” is correct. The electrons in 2p orbitals of two carbons overlap to form a π bond. When addition happens, it is usually syn addition (same side of p orbital), and thus the substituents on two carbons “overlaps” with each other. This is the eclipsed conformation (high energy, not favored). It will then quickly change to staggered conformation.
True
Statement “sp-, sp2-, and sp3-hybridized orbitals can sometimes form π-bonds”
is incorrect. Only unhybridized p orbitals can form π bond because geometrically they are perpendicular to all the hybridized orbitals.
What makes up a pi bond
Sideways Overlap of p orbitals not sp or s!!!
Hybrid orbitals containing two electrons (a lone pair) cannot participate in forming covalent bonds. This is false. For example, the oxygen atom in water is sp3 hybridized, with each lone pair of electrons on oxygen occupying an sp3 hybrid orbital. The lone pair is attracted to and can form a covalent bond with a carbocation, as shown below.
Yeah false
The px, py, and pz orbitals are all at 90° angles from one another — like the x, y, and z axes in 3D space.
Since the σ bonds used px, the π bonds are using py and pz, which are at 90° to each other.
Remember that!
Acid-catalyzed hydration of an alkene goes through the least highly substituted carbocation intermediate” is false. Based on the stability principle of carbocation, the most highly substituted intermediate state will be preferred because it has the lowest activation energy. |
Remember
In the formation of a Grignard reagent from an alkyl halide, the carbon atom bearing the halide is reduced.
This statement is true. A Grignard reagent is formed by mixing an alkyl halide with magnesium in diethyl ether. In the original alkyl halide (R3C–X), the C has a δ+ charge and the X (halogen) has a δ– charge. When Mg inserts between C and X, it generates R3CMgX, in which Mg has a 2+ charge, X has a 1– charge, and C has a 1– charge. Thus, the oxidation state of carbon has changed from δ+ to 1– => reduction.
Remember
Hydrogenation of alkynes using Lindlar’s catalyst leads to Z - alkenes
Remember!
Hydrogenation occurs via syn addition, in which both hydrogen atoms add to the same face of the alkene, which is a ring in this exampl
Remember that
Combo of one s and three p makes 4 orbitals of equal energy
An isolated p orbital is higher in energy than an sp3 hybrid orbital
carbon typically forms four bonds, and thus has four bonding orbitals, and these bonds are typically single bonds. These bonds are formed through sp3 hybridization, where the four orbitals hybridize from the single 2s orbital and the three 2p orbitals of the carbon atom
In alkenes the unhybridized p-orbitals form a -bond and force the substituents spacedi ted alias atons to become elin sp hybridized