L18 transplantation
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Why are transplanted organs rejected?
Rejection occurs because the recipient’s adaptive immune system recognises a non-genetically identical donor’s MHC molecules as foreign, triggering T-cell responses.
What are alloantigens?
Alloantigens are antigens that differ between individuals of the same species—mainly MHC molecules, which drive strong alloreactive T-cell responses.
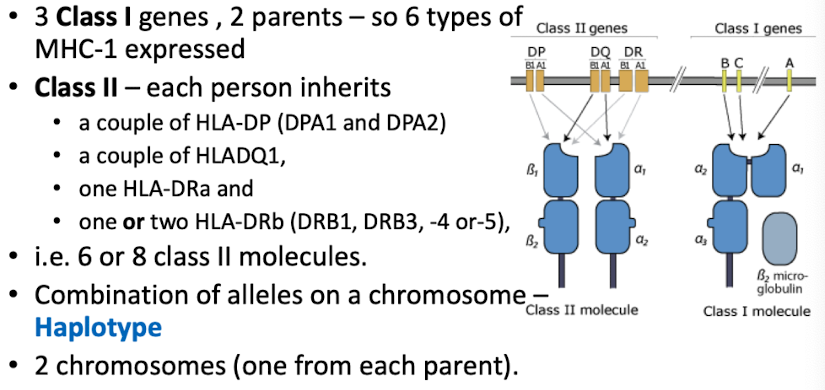
What features of MHC explain strong rejection responses?
Highly polymorphic—no two individuals share identical MHC alleles.
Co-dominant expression—both maternal and paternal alleles are expressed.
Leads to high likelihood of mismatches between donor and recipient.
Allogenic individual
unrelated individuals
What are minor histocompatibility antigens?
Non-MHC proteins that differ between donor and recipient; elicit slower rejection even with perfect MHC matching (e.g., 1/3 encoded on Y chromosome).
What is the difference between nucleated cell transplant and red blood cell trasplant?
Nucleated cells transplant: T cell responses to MHC molecules always trigger a response
• Matching MHC type
• Perfect matching
Red blood cell transplant – express tiny amounts of MHC class 1 and no MCH class II
What is direct allorecognition and when does it occur?
Recipient T cells directly recognise intact donor MHC molecules without processing by donor APCs i.e. DC
Organ graft contains donor APCs which stimulate a response
Leave transplanted tissue and migrate from the graft to lymph nodes → T cell activation
Drives acute, early rejection.
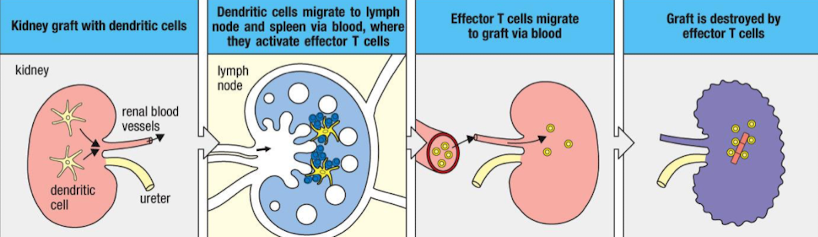
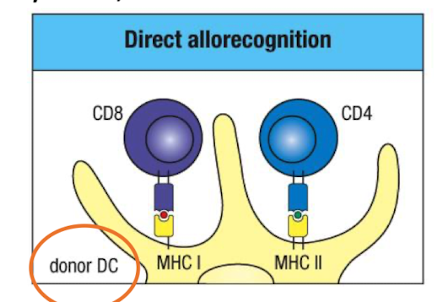
Outline the steps of direct allorecognition
Donor organ contains donor APCs.
Donor APCs migrate to recipient secondary lymphoid organs.
They activate recipient alloreactive T cells.
Effector T cells return to graft and kill donor cells → acute rejection.
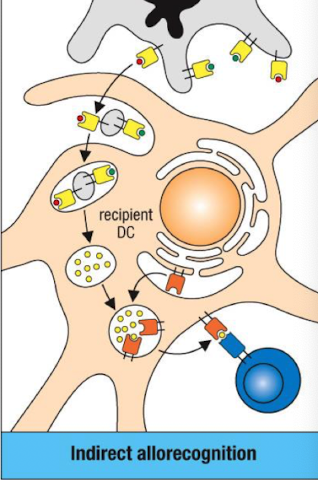
What is indirect allorecognition?
Recipient APCs process donor MHC molecules into peptides and present them on self MHC to recipient T cells.
Contributes to chronic rejection
Outline the steps of indirect allorecognition.
Donor APCs die → release vesicles with allogeneic MHC I/II.
Recipient DC endocytose donor MHC.
Peptides presented on recipient MHC (HLA) to activate T cells.
Why are T cells the main mediators of graft rejection?
T cells respond strongly to foreign MHC (even more strongly than to foreign peptides). Thus, any nucleated cell graft expressing MHC I triggers T-cell activation.
Distinguish first-set and second-set rejection.
First-set: Primary T-cell response; slower.
Second-set: Faster and stronger due to T-cell memory.
What causes hyperacute rejection & how is it prevented?
Pre-existing antibodies against donor antigens (e.g., anti-ABO or anti-HLA).
Prevented by testing for pre-formed antibodies via flow cytometry/ELISA.
What characterises acute rejection?
T-cell mediated, rapid.
Driven mainly by direct allorecognition of donor MHC on donor APCs.
What drives chronic rejection?
Long-term, slow graft deterioration, associated with indirect allorecognition
Persistent low-grade T-cell and antibody responses, fibrosis, vasculopathy.
(Mechanism implied from indirect pathway.)
Why is ABO matching required?
ABO matching is required to prevent hyperacute-Ab-mediated rejection due to pre-existing Ab against incompatible blood group antigens
Matching minimises risk of immediate antibody-mediated rejection, especially for kidney / heart transplants
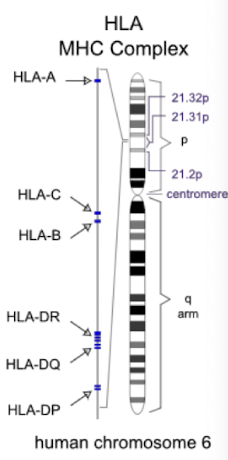
What does tissue typing involve?
Matching donor and recipient HLA-A, HLA-B, and HLA-DR; better matching strongly correlates with higher graft survival.
*HLA = human leukocyte antigen, often people with similar ethnic background may have more closely related HLA types, enhancing compatibility.
Why screen recipient serum before transplantation?
To detect pre-formed anti-donor HLA antibodies using fluorescent bead assays or crossmatching to prevent hyperacute rejection
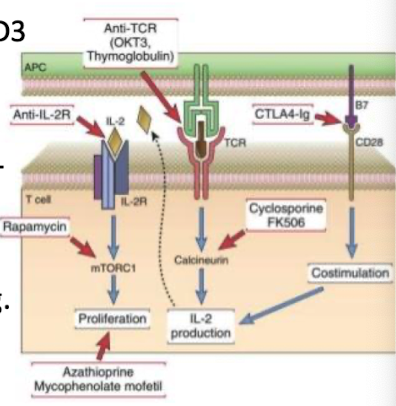
How do cyclosporine and FK506 prevent rejection?
Inhibit calcineurin → block IL-2 transcription → prevent T-cell activation
calcineurin: calcium and calmodulin-dependent enzyme crucial for activating T cells in the immune system by dephosphorylating the transcription factor NFAT
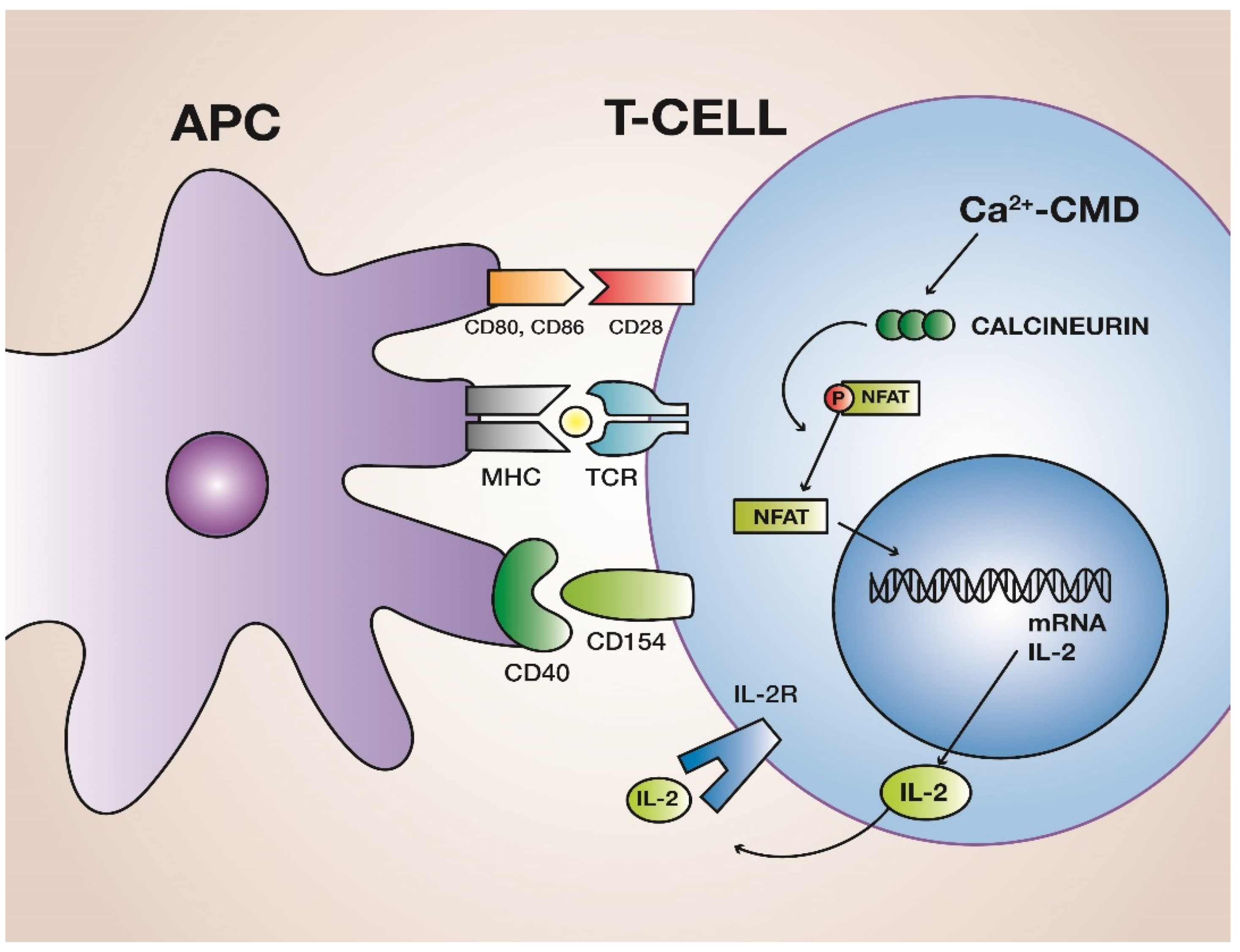
How does rapamycin work?
Inhibits mTOR, blocking IL-2-driven T-cell proliferation.
mTOR is a protein kinase involved in cell growth and metabolism regulation.
How does mycophenolate mofetil work?
Blocks lymphocyte-specific IMP dehydrogenase → prevents guanine synthesis → kills proliferating T cells
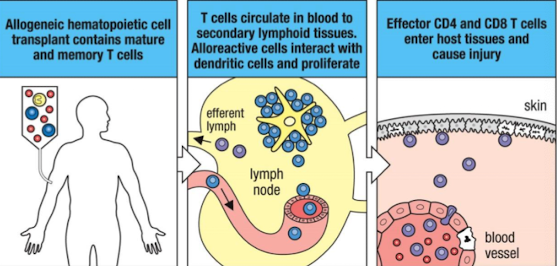
What is GVHD?
Graft-versus-host disease (GVHD) is a condition that occurs when donated bone marrow or peripheral blood cells attack the recipient's body.
It can arise after a transplantation, particularly in allogeneic transplants, and may cause symptoms such as skin rash, liver dysfunction, and gastrointestinal problems.
Where is HSC therapy used?
Primarily in the treatment of blood disorders, including leukemias, lymphomas, and certain genetic disorders.
Also utilised in the transplantation process to restore bone marrow function in patients undergoing chemotherapy or radiation.
Why is GVHD more serious in HSC transplants?
GVHD is more serious in hematopoietic stem cell (HSC) transplants because HSCs are capable of generating a full immune system, which can attack the host tissues more aggressively, leading to more severe symptoms.
How do Tregs influence graft outcomes?
Depleting CD25+ Tregs accelerates GVHD; supplementing them with fresh/ ex vivo Treg cells reduces it.
CD8⁺CD28⁻ T cells also help maintain allograft tolerance by interfering with CD4 T cell activation by APCs
Why is the fetus tolerated despite paternal MHC?
Foetus - paternal MHC and minor
histocompatibility Ag- different from
the mother’s: However tolerance is mediated by:
Placental sequestration of fetal tissue
Placenta inhibits nutrient depletion (tryptophan)
Immunosuppressive cytokines (TGF-β, IL-10)
Examples of biologics used in immunosuppression?
Anti-CD3 (TCR) (OKT3): removes T cells from circulation
CTLA4-Ig: blocks CD28–B7 co-stimulation.
Corticosteroids: reduce inflammatory rxns to allografts