pH Scale
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
acid
Substance that has a pH less than 7 and donates a hydrogen ion (H+, a proton) to a solution. Few OH⁻ and many H⁺. Turns cabbage juice indicator pink/red/orange.
base
Substance that has a pH greater than 7 and donates a hydroxide ion (-OH) to a solution. Many OH⁻ and few H⁺. Turns cabbage juice indicator blue/green/yellow.
neutral
Substance with a pH of 7 and equal amounts of hydrogen (H+) and hydroxide (-OH) ions. Does not change the color of cabbage juice indicator (stays purple).
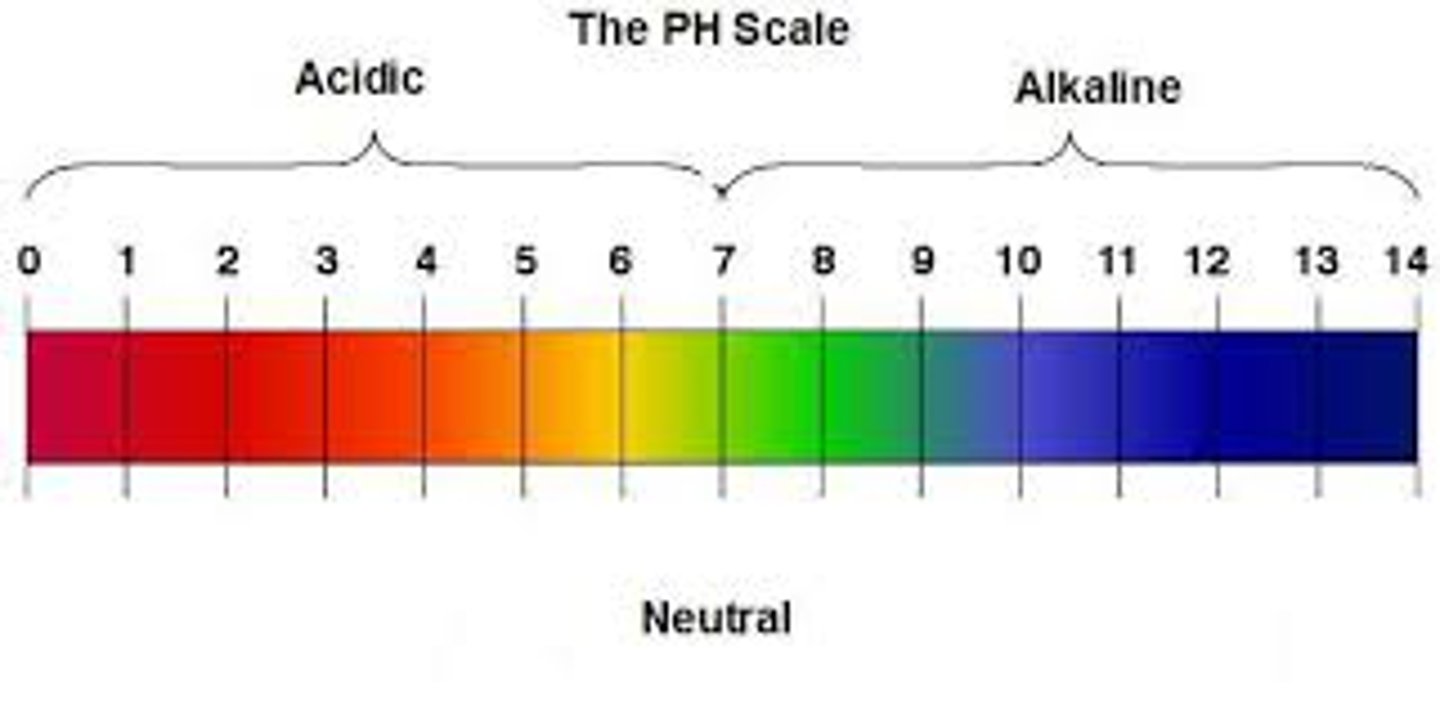
pH scale
A system for measuring the amount of hydrogen ions (H+) in a solution to determine how acidic or basic it is on a range of 0-14.
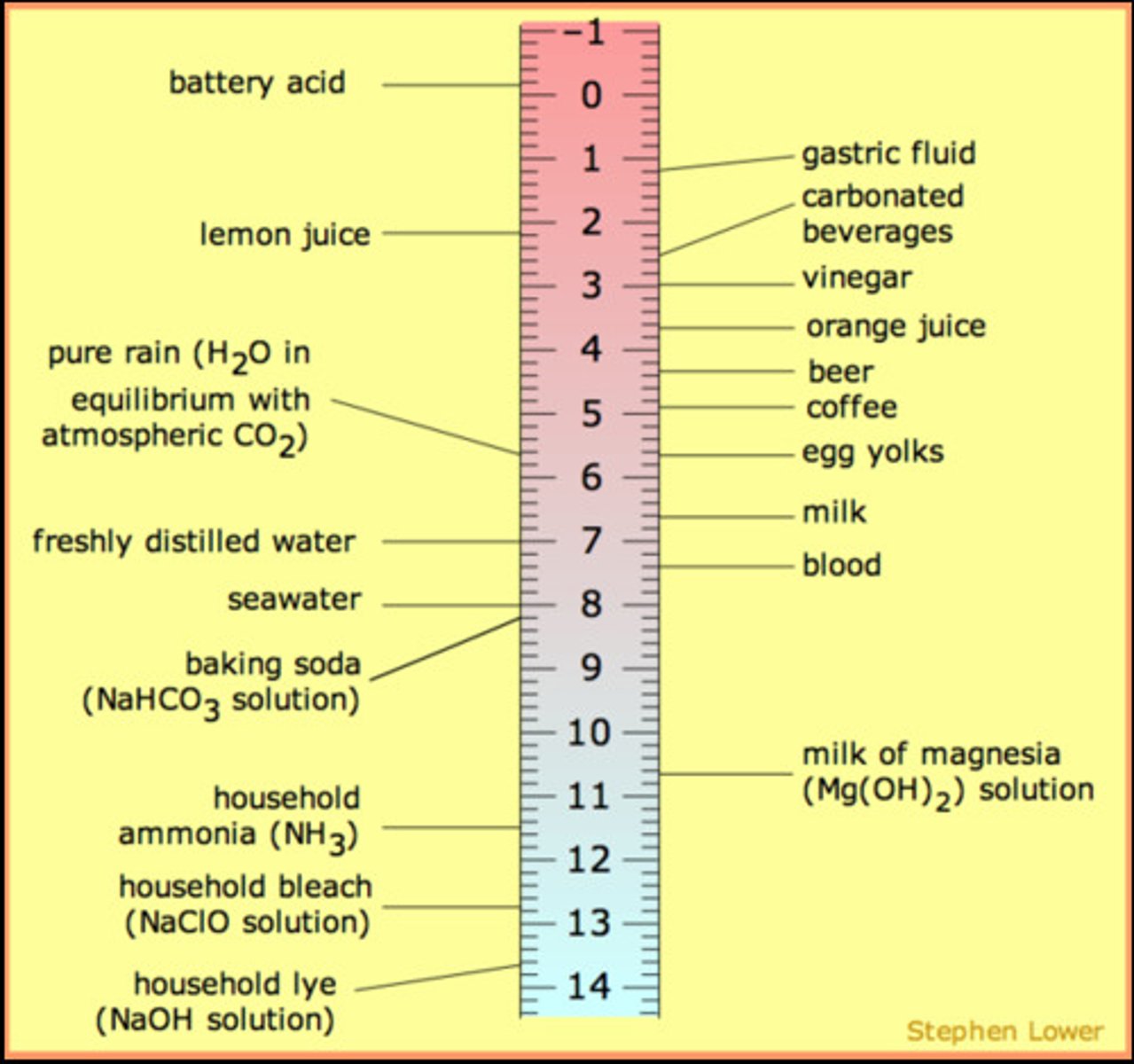
examples of weak acids
urine, soda (carbonated water), rainwater, coffee, orange juice
examples of stronger acids
lemon juice, gastric (stomach) juices, tomato juice, vinegar
examples of stronger bases
bleach, ammonia
examples of weak bases
blood, tears, baking soda, soap
neutralization
A reaction between an acid and base, bringing the solution closer to neutral.