D BLOCK
1/43
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
D-block elements are called transition elements. Justify this statement
Because their properties are transitional between highly reactive metals of s block and elements of p block which are mostly non metals.
What are transition elements?
IUPAC defines transition metal as an element whose atom has an incomplete d sub shell or which can give rise to cations with an incomplete d sub shell.
General Electronic configuration of D-block
3d series is from …………… to ………………
Scandium to Zinc
4d series is from …………… to ………………
Yttrium to Cadmium
5d series is from …………… to ………………
Lanthanum to Mercury
Why there is a slight variation in the atomic radii from Cr to Cu?
As we move from Sc to Zn in 3d series the extra electrons are added to the 3d orbitals, the added 3d electrons only partially shield the increased nuclear charge and hence the effective nuclear charge increases slightly.
However, the extra electrons added to the 3d sub shell strongly repel the 4s electrons and these two forces operating in opposite direction tend to balance each other, leading to a constancy in atomic radii.
At the end of the series, d – orbitals of Zinc contain 10 electrons in which the repulsive interaction between the electrons is more than the effective nuclear charge and hence, the orbitals slightly expand and atomic radius slightly increases.
Expected and Actual electronic configuration of chromium and copper.
What are the metallic behavior of d-block elements?
All the transition elements are metals.
Like other metals, transition metals are good conductors of heat and electricity.
Unlike the metals of Group-1 and group-2, all the transition metals except group 11 elements are hard.
Why transition elements have high melting point?
Due to presence of unpaired electron in their atoms, transition elements in general, have stronger interatomic interaction and hence stronger bonding between atoms. As a result they have high melting point.
d-block elements have variable oxidation state. Why?
The first transition metal Scandium exhibits only +3 oxidation state, but all other transition elements exhibit variable oxidation states by losing electrons from (n-1)d orbital and ns orbital as the energy difference between them is very small.
Write a note on the oxidation state of 3d series.
The first transition metal Scandium exhibits only +3 oxidation state, but all other transition elements exhibit variable oxidation states by losing electrons from (n-1)d orbital and ns orbital as the energy difference between them is very small.
At the beginning of the series, +3 oxidation state is stable but towards the end +2 oxidation state becomes stable.
The number of oxidation states increases with the number of electrons available, and it decreases as the number of paired electrons increases.
Hence, the first and last elements show less number of oxidation states and the middle elements show more number of oxidation states.
For example, the first element Sc has only one oxidation state +3; the middle element Mn has six different oxidation states from +2 to +7. The last element Cu shows +1 and +2 oxidation states only.
Ru and Os have highest oxidation state in which compounds?
The highest oxidation state of 4d and 5d elements are found in their compounds with the higher electronegative elements like O, F and Cl.
Example: RuO4 , OsO4
Which metal in the 3d series exhibits +1 oxidation state most frequently and why?
Copper has an electronic configuration of [Ar]3d104s1.
It readily loses one electron from its 4s orbital to give a stable 3d10 electronic configuration.
When one electron is lost, the configuration becomes most stable due to fully filled d^(10) configuration.
Define Standard electrode potential
Standard electrode potential is the value of the standard emf of a cell in which molecular hydrogen under standard pressure ( 1atm) and temperature (273K) is oxidised to solvated protons at the electrode.
If the standard electrode potential (E0) of a metal is large and negative, the metal is a powerful reducing agent, because it loses electrons easily.
Write note on diamagnetism. Give example.
Materials with no elementary magnetic dipoles are diamagnetic in nature.
In other words, a diamagnetic species is a species in which all the electrons are paired.
Diamagnetic materials are repelled by magnetic fields because in the presence of external magnetic field, a magnetic induction is introduced in the material which generates weak magnetic field that opposes the applied field.
Write note on paramagnetism. Give example.
Paramagnetic solids having unpaired electrons possess magnetic dipoles which are isolated from one another.
In the absence of external magnetic field, the dipoles are arranged in random directions and hence the solid shows no net magnetism.
But in the presence of external magnetic field, the dipoles are aligned parallel in the direction of the applied field and therefore, they are attracted by an external field.
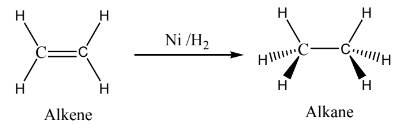
Most of the transition metals act as catalyst. Justify this statement.
Many industrial processes use transition metals or their compounds as catalysts.
Transition metals have energetically available d orbitals that can accept electrons from reactant molecule or form bonds with reactant molecule using their d electrons.
For example, in the catalytic hydrogenation of an alkene, the alkene bonds to an active site by using its π electrons with an empty d orbital of the catalyst.
The σ bond in the hydrogen molecule breaks, and each hydrogen atom forms a bond with a d electron on an atom in the catalyst.
The two hydrogen atoms then bond with the partially broken π -bond in the alkene to form an alkane.
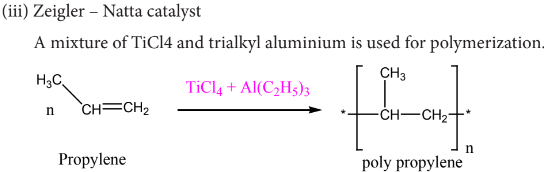
What is Zeigler – Natta catalyst?
Hume-Rothery rule
According to Hume-Rothery rule to form a substitute alloy
the difference between the atomic radii of solvent and solute should be less than 15%.
Both the solvent and solute must have the same crystal structure and valence
their electronegativity difference must be close to zero.
D-block elements readily form Alloy. Give reason.
D block elements readily form alloys as their atomic sizes are similar and one metal atom can easily replace another metal atom from its crystal lattice to form an alloy.
What are interstitial compounds?
An interstitial compound or alloy is a compound that is formed when small atoms like hydrogen, boron, carbon or nitrogen are trapped in the interstitial holes of a metal lattice.
They are usually non-stoichiometric compounds.
Transition metals form a number of interstitial compounds such as TiC, Zr1.92H , Mn4N etc.
The elements that occupy the metal lattice provide them new properties.
(i) They are hard and they show electrical and thermal conductivity
(ii) Their melting points is higher than those of pure metals
(iii) Transition metal hydrides are used as powerful reducing agents
(iv) Metallic carbides are chemically inert.
d-block elements readily form complexes. Give reason
Transition metal ions are small, highly charged and they have vacant low energy orbitals to accept an electron pair donated by other species. Due to these properties, transition metals form large number of complexes.
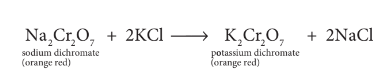
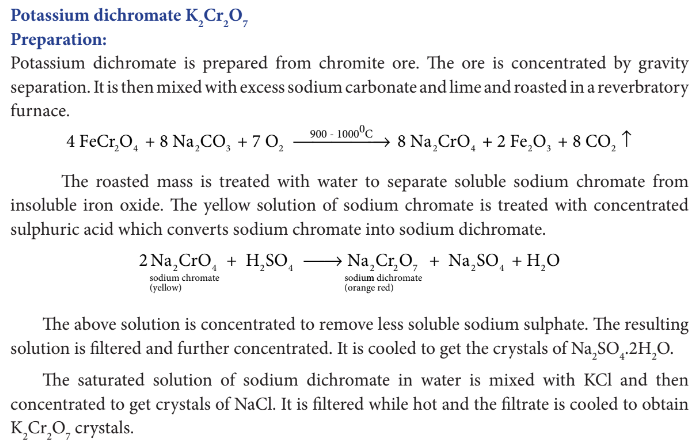
Describe preparation of potassium dichromate.
.How does potassium dichromate decompose on heating?
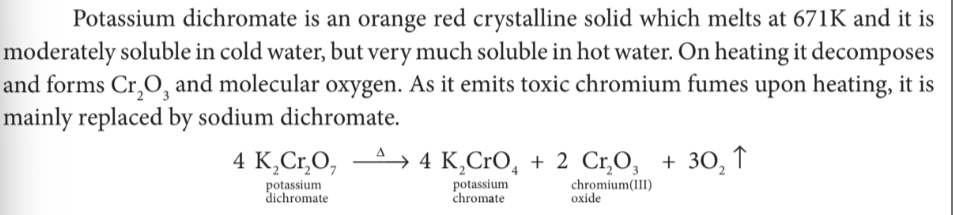
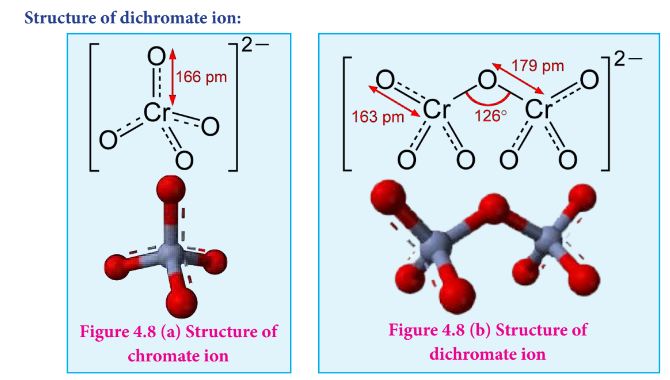
Draw the structure of chromate, dichromate ions
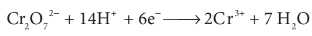
How does potassium dichromate act as oxidizing agent?
Potassium dichromate is a powerful oxidising agent in acidic medium.
There is a change in the oxidation
state of chromium from Cr6+ to Cr3+.


Explain chromyl chloride test.
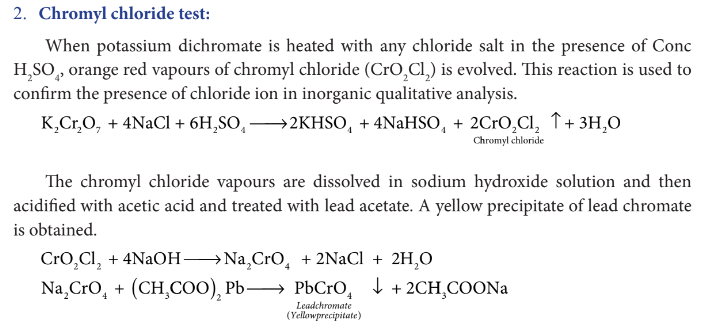

Mention the uses of potassium dichromate
1. It is used as a strong oxidizing agent.
2. It is used in dyeing and printing.
3. It used in leather tanneries for chrome tanning.
4. It is used in quantitative analysis for the estimation of iron compounds and iodides.
Which is predominant: Chromate or Dichromate ion?
In aqueous solution, chromate and dichromate ions are interconvertible.
In an alkaline solution chromate ion is predominant and in acidic solutions dichromate ion becomes predominant.
How will you prepare potassium permanganate?
What is Bayer’s reagent?
Cold dilute alkaline KMnO4 is known as Baeyer’s reagent.
It is used to oxidize alkenes into diols.
For example, ethylene can be converted into ethylene glycol.
This reaction is used as a test for unsaturation.

Uses of Potassium Permanganate
1. It is used as a strong oxidizing agent.
2. It is used for the treatment of various skin infections and fungal infections of the foot.
3. It used in water treatment industries to remove iron and hydrogen sulphide from well
water.
4. It is used as Bayer’s reagent for detecting unsaturation in an organic compound.
5. It is used in quantitative analysis for the estimation of ferrous salts, oxalates, hydrogen peroxide and iodides.
Justify the position of lanthanoids and actinoids in the periodic table.
The actual position of Lanthanoids in the periodic table is at group number 3 and period number 6.
However, in the sixth period after lanthanum, the electrons are preferentially filled in inner 4f sub shell and these fourteen elements following lanthanum show similar chemical properties. Therefore these elements are grouped together and placed at the bottom of the periodic table. This position can be justified as follows.
1. Lanthanoids have general electronic configuration [Xe] 4f1-14 5d1-10 6s1-2
2. The common oxidation state of lanthanoids is +3
3. All these elements have similar physical and chemical properties.
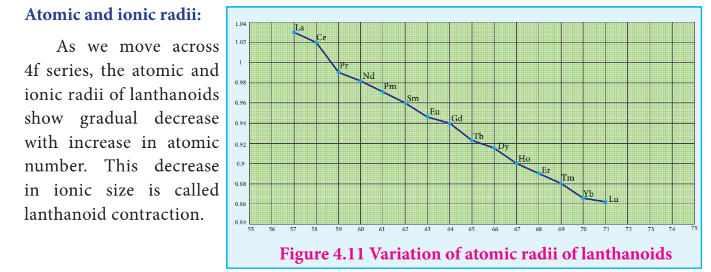
Causes of Lanthanoid contraction.
As we move from one element to another in 4f series ( Ce to Lu) the nuclear charge increases by one unit and an additional electron is added into the same inner 4f sub shell.
But since 4f sub shell has a diffused shape, the shielding effect of 4f electrons is relatively poor
Therefore, with increase in nuclear charge, the valence shell is pulled slightly towards nucleus.
As a result, the effective nuclear charge experienced by the 4f electrons increases and the size of Ln3+ ions decreases.
Consequences of Lanthanoid contraction.
Basic Nature
As we from Ce3+ to Lu3+ , the basic character of Ln3+ ions decrease.
Due to the decrease in the size of Ln3+ ions, the ionic character of Ln OH − bond decreases (covalent character increases) which results in the decrease in the basic nature.
Similarities among lanthanoids:
In the complete f - series only 10 pm decrease in atomic radii and 20 pm decrease in ionic radii is observed.
Because of this very small change in radii of lanthanoids, their chemical properties are quite similar.
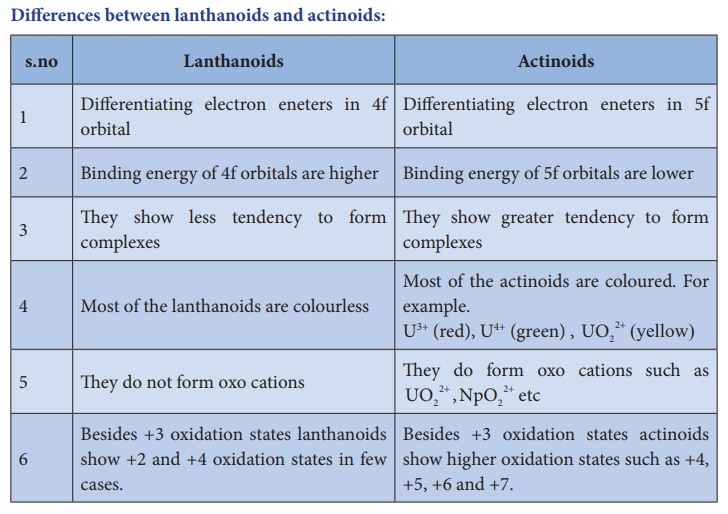
Differences between Lanthanoids and Actinoids
What is Lanthanoid Contraction?
General electronic configuration of actinoids

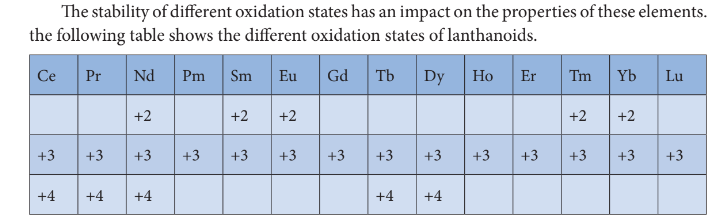
Oxidation State of Lanthanoids

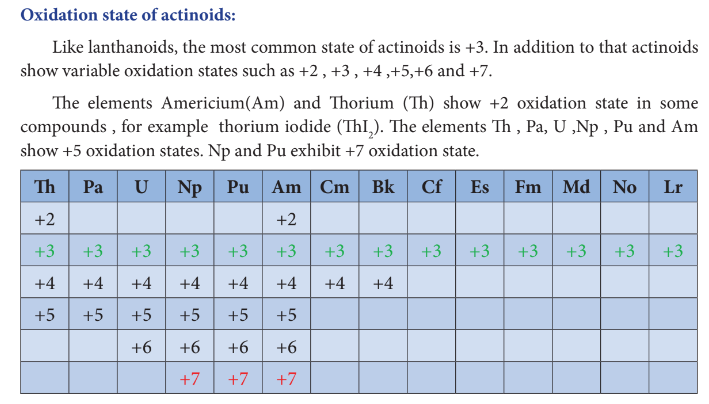
Oxidation State of Actinoids