Chemical Reagent List
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
HX (Hydrohalogenation)
An addition reaction in which a Hydrogen (H) and a Halogen (X) are added across an alkene or alkyne.
Major product: Halogen added to more substituted side
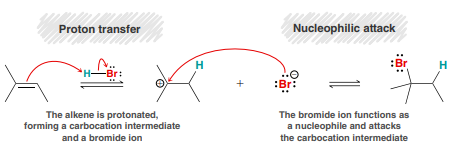
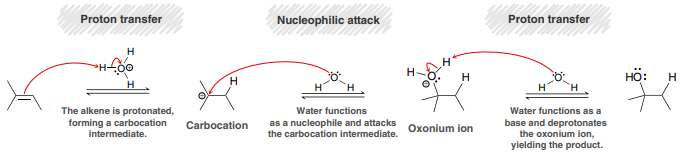
H2O, H2SO4 (Acid-Catalyzed Hydration)
An addition reaction in which water (H and OH) is added across an alkene.
Major product: OH added to more substituted side
Hg(OAc)2, H2O
NaBH4
(Oxymercuration-Demercuration)
An addition reaction in which water (H and OH) is added across an alkene, while preventing carbocation rearrangements.
Major product: OH added to more substituted side
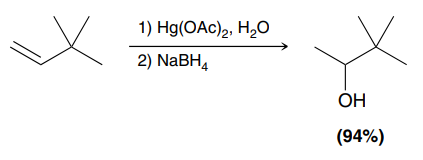
BH3*THF
H2O2, NaOH
(Hydroboration-Oxidation)
An addition reaction in which water (H and OH) is added across an alkene.
Major product: OH added to less substituted side; syn addition
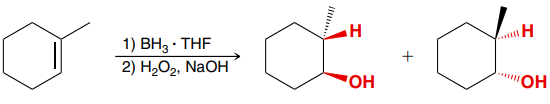
H2, Pt (Catalytic Hydrogenation)
An addition reaction in which H2 (H and H) is added across an alkene.
Product: Syn addition
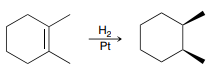
X2 (Halogenation)
An addition reaction in which X2 (X and X) is added across an alkene
Product: Anti addition
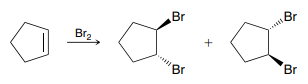
X2, H2O (Halohydrin Formation)
An addition reaction in which X and OH are added across an alkene
Product: Anti addition, Markovnikov
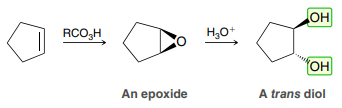
MCPBA - or any peroxyacid
Formation of an epoxide
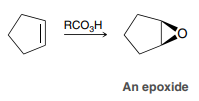
MCPBA
H3O+
(Anti Dihydroxylation)
Formation of a trans-diol
Product: Anti addition
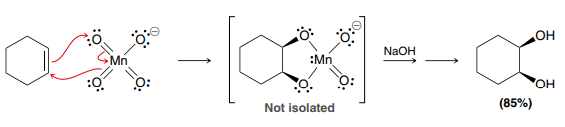
OsO4
NaHSO3/H2O
OR
KMnO4, NaOH, cold
(Syn Dihydroxylation)
Formation of a diol
Product: Syn addition
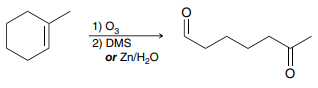
O3
DMS
(Ozonolysis)
A reaction in which a carbon-carbon double or triple bond is cleaved when an alkene or alkyne reacts with ozone (O3)
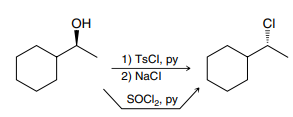
SOCl2
Primary and secondary alcohols can react with SOCl2 via an SN2 process, replacing the hydroxyl group with a chlorine, and resulting in an inversion of configuration
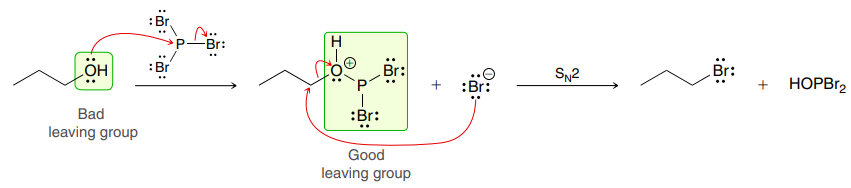
PBr3
Primary and secondary alcohols can react with PBr3 via an SN2 process, replacing the hydroxyl group with a bromine, and resulting in an inversion of configuration
NaNh2/NH3 (l)
H2O
Used to prepare alkynes from alkyl dihalides (only use H2O if alkyne is terminal)
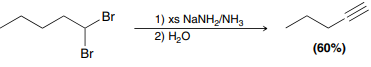
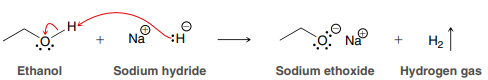
NaH
Used to deprotonate an alcohol
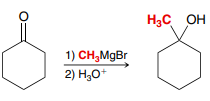
BrMgX
H3O+
(Grignard Reaction)
Used to prepare an alcohol from a ketone/aldehyde/ester
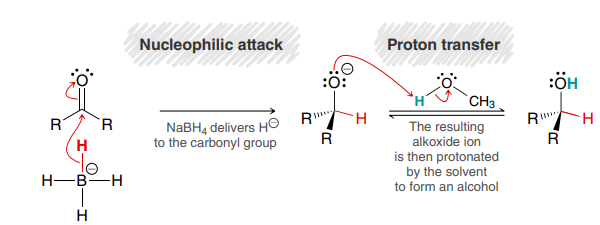
NaBH4, protic solvent
Used to reduce ketones or aldehydes to alcohols
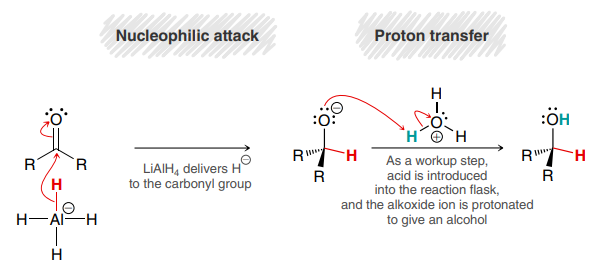
LiAlH4
H3O+ (or H2O)
Used to reduce ketones or aldehydes to alcohols
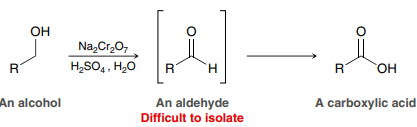
Na2Cr2O7, H2SO4, H2O (Chromium-based Oxidation)
Used to convert a primary alcohol to a carboxylic acid or a secondary alcohol to a ketone
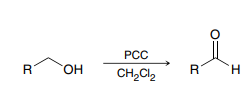
PCC, CH2Cl2
Used to convert a primary alcohol to an aldehyde or a secondary alcohol to a ketone
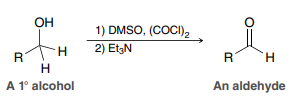
1) DMSO, (COCl)2
2) Et3N
(Swern Oxidation)
Used to convert a primary alcohol to an aldehyde
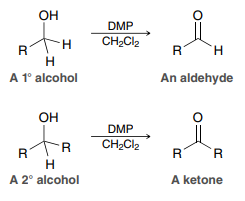
DMP, CH2Cl2 (DMP Oxidation)
Used to convert a primary alcohol to an aldehyde or a secondary alcohol to a ketone