Chemical Formulas
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Molecular Formula
Gives the actual number of atoms of each element in a compound. See image for example
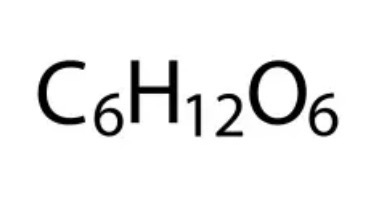
Compound
Chemical consisting of more than one atom (they are stuck together). EX: water, H(small 2)O. 2 Hydrogen atoms and 1 Oxygen atom.
Empirical Formula
Gives the smallest whole number ratio in which atoms of each element are present in the compound.
To Convert from Molecular Formula to Empirical…
Look for the largest common denominator to get the smallest whole number. EX: Molecular formula C4H10 (4:10 ratio, 4&10 largest common denominator is 2, therefore,) its Empirical Formula is now C2H5, 2:5 ratio.
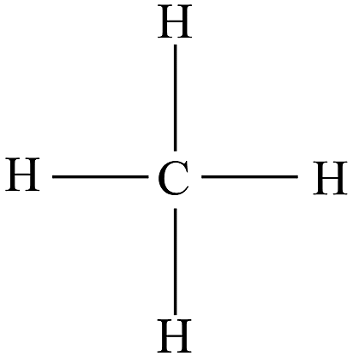
Structural Formula
Gives the actual number of atoms of each element in a compound AND how they are connected to each other.
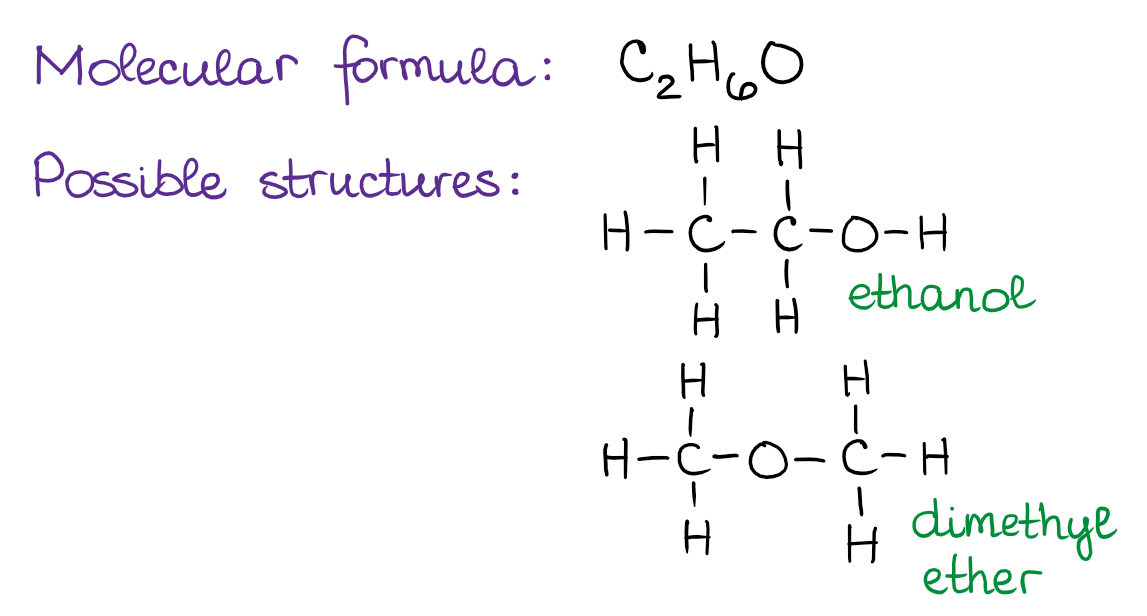
Isomers
Compounds with the same molecular formula but a different structural formula. (See image for example of structural isomers).
Spatial Isomers
Structural Isomers with the same connection to the atoms but different 3D position (mirror image of each-other, like your hands).