Topic #3 - Protein Function (oxy. binding proteins)
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
Molecular Oxygen (O2)
O2 exists as a molecule (not an element)
Human Body consumes 500g O2 a day (Issue: O2 not easily transported/ 4.1 g dissolved in blood plasma)
Solution: Oxygen Binding proteins
2 main ones: Myoglobin & Hemoglobin
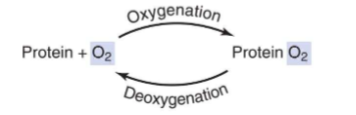
What allows to bind to molecular O2?
Heme
Prosthetic group (important for Heme)
tightly bound chemical moiety necessary for some proteins’ functions (Fe has prosthetic group)
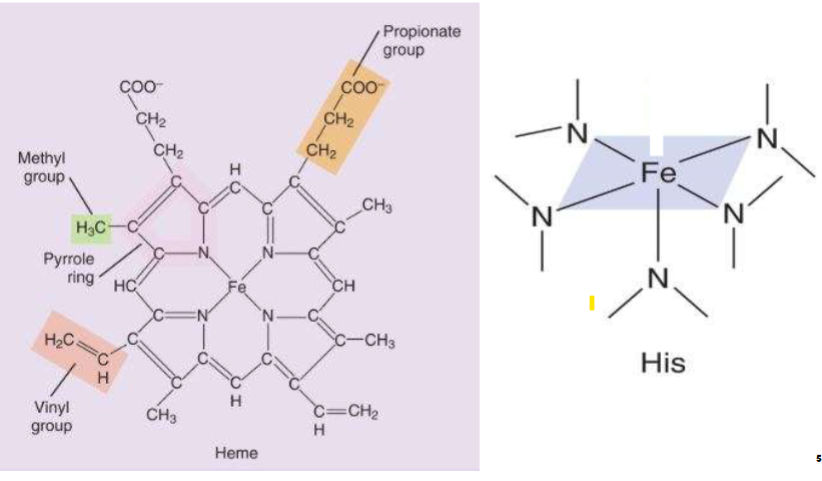
Heme info
II. Composed of an organic molecule called
protoporphyrin IX
- A. Four 5-membered pyrrole rings containing methyl,
vinyl, & propionate side chains
- B. Forms 4 coordinate covalent bonds (covalent bond in which
both e- of bond are supplied by 1 atom) to an Fe ion
-1. Fe ion can form a 5th coordinate covalent bond
with proximal histidine N atom
Heme Structure
-Heme has an Iron (Fe)
leaving top part open for O2 binding (on right side)
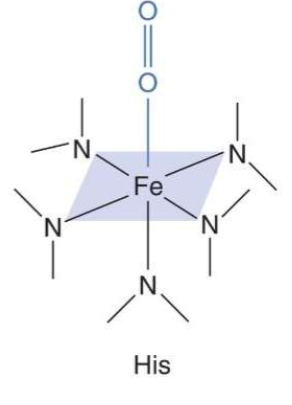
What is Function of Heme?
. O2 binding moiety
A. No AA side chain possesses the ability to reversibly bind O2
B. Heme can & does
1. Fe core forms a reversible 6th coordinate covalent
bond with O2 6
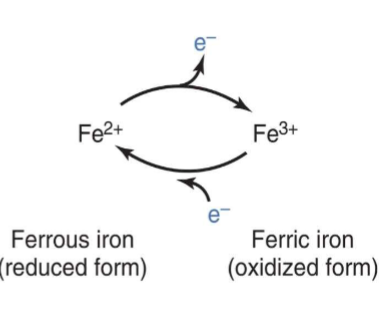
Iron (Fe) Oxidation States (for Heme)
Can easily go back and forth between states
I. Heme Fe can exist in 2 ionic forms (i.e., 2 oxidation
states)A. Ferrous form (Fe2+; reduced form)
B. Ferric form (Fe3+; oxidized
form)C. ONLY ferrous form
can bind O2
Heme in Blood
I. Conjugated C=C double bond
system of heme group gives
blood its colorA. When heme is
oxygenated, color of blood
is redB. When heme is
deoxygenated, color of
blood is red-purple
Myoglobin (Mb)
I. Tightly packed globular protein found in muscle tissue
II. Functions as a short-term storage depot of O2
in muscles
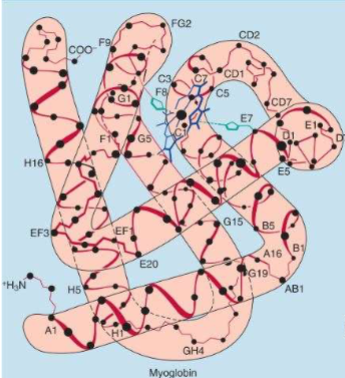
Myglobin Structure
I. Contains 1 tightly bound heme group, surrounded by 8 -helices
A. This group binds O2; globin chain functions to keep Fe in ferrous state)
II. Contains no disulfide bonds
A. 3° structure is held together by
noncovalent interactions (particularly hydrophobic
interactionsDont memorize structure
Hemoglobin (Hb)
Each protein capable of binding to 4 o2
I. Found only in erythrocytes (red blood cells, or RBCs; makes up ~33% of their cytoplasm) & their precursors in the bone marrow
II. Heterotetramer composed of 4 polypeptide chains,
each with its own heme group (held together by hydrogen bonds &
salt bridges, but no disulfide bonds)A. Major adult Hb (HbA): α2β2 (makes up ~ 97% of adult Hb)
B. Minor adult Hb (HbA2): α2β2 (makes up ~ 2 – 3% of adult Hb)
C. Fetal Hb (HbF): α2γ2 (major Hb present during 2nd & 3rd trimesters of pregnancy; makes up < 2% of Hb in adults)
Each Heme group can bind to 4 O2
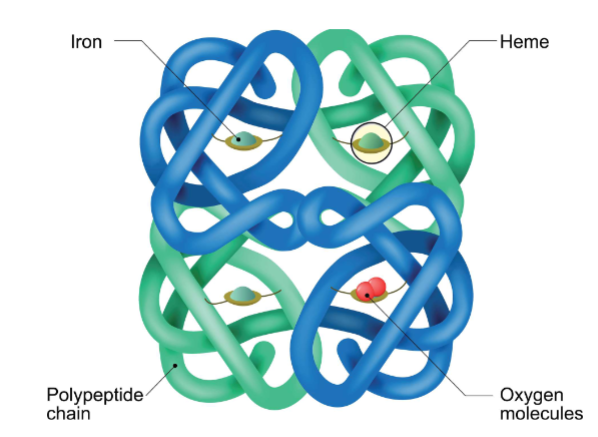
Conformations of Hemoglobin
I. O2 binding changes hemoglobin’s 4° structure
A. In absence of O2 (deoxyhemoglobin), each polypeptide chain is in T conformation (tense or taut)
1. Due to formation of 8 salt bridges & many hydrogen bonds between subunits
B. O2 binding breaks all 8 salt bridges & causes formation of new hydrogen bonds
1. Causes polypeptide chains to adopt R conformation (relaxed)
To go through these state molecular binding is necessary
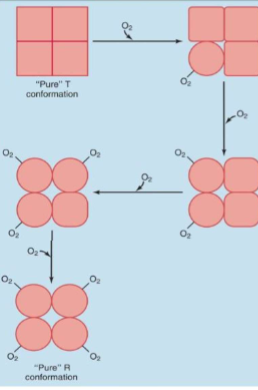
Cooperativity of O2 Binding
Example of Pos. Cooperativity
I. 1st O2 binding event is difficult (because Hb subunits are in T conformation; heme groups are not optimally positioned for O2 binding)
A. Once O2 binding occurs, it converts that polypeptide chain into R conformation & destabilizes its interactions with other subunits, which alters their conformations
1. Moves other heme groups into more optimal positions
i. Each subsequent O2 binding event is easier than the next because of these changes
Cooperativity of O2 Binding (2)
I. Each O2 binding converts binding subunit into R conformation
A. R conformation binds O2 150 – 300x more tightly than T conformation
II. Thus, O2 binding to hemoglobin displays positive cooperativity
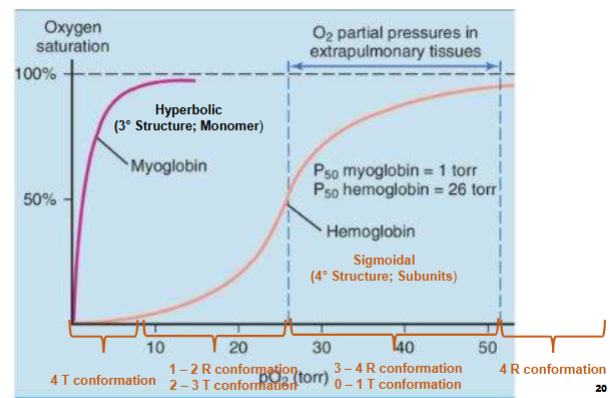
O2- Binding Curve
I. Describes fractional saturation of heme groups at varying pO2 (partial pressure of O2)A. p50 is the pO2 required to oxygenate ½ of all heme groups present
1. For Mb, p50 is ~ 1 torr
2. For Hb, p50 is ~ 26 torr
i. Mb binds much tighter to O2 than Hb
What is likely state of Hemoglobin (Looking at curves for Hemoglobin & Myoglobin)
x-axis partial pressure of subunits in torr
y-axis o2 saturation
1) Myo. is a monomer, has a tertiary structure, 1 site in o2 binding (hyperbolic curve meaning)
2) Hemo. is a sigmodial curve, its not a monomer, quarternary structure, has cooperativity (binding of ligand in diff. sites effects binding of ligand in other sites)
when at low O2 conc. all 4 subunits at T state
from p10-p50 have 1-2 subunits in R confirmation & 2 in T confirmation
Past p50 to plateau seeing more subunits in R confirmation, less in T
At plateau all subunits in R confirmation (meaning saturated all sites are bound to O2)
Hb Saturation
Bc of pos. cooperativity & tight R conformation O2 binding, Hb efficiency as O2 transporter increased
A. Hb is ~ 96% saturated in lung capillaries (because of high pO2 of 90 torr)
B. Hb is ~ 33% saturated in capillaries of active skeletal muscle (because of low pO2 of 20 torr)
1. Hb actively releases O2 into active skeletal muscles
2. Less O2 is released in other body tissues
i. Venous blood remains 60 – 70% oxygenated
Hb Regulation by 2,3-BPG (how we control hemoglobin)
2,3-bisphosphoglycerate
A. [2,3-BPG]RBC ≈ [Hb]
B. Binds exclusively to T conformation of Hb, stabilizing it
1. In presence of 2,3-BPG, T conformation predominates over R
conformation
2. Decreases Hb O2-binding
affinity
Makes hemoglobin’s binding ability harder (keeps subunits in T state)
![<ul><li><p><span style="color: rgb(0, 0, 0);"><strong>2,3-bisphosphoglycerate</strong></span></p><ul><li><p><span style="color: rgb(0, 0, 0);">A. [2,3-BPG]RBC ≈ [Hb]</span></p></li><li><p><span style="color: rgb(0, 0, 0);">B. Binds exclusively to T conformation of Hb, stabilizing it </span></p><ul><li><p><span style="color: rgb(0, 0, 0);">1. In presence of 2,3-BPG, T conformation predominates over R</span></p><p><span style="color: rgb(0, 0, 0);">conformation</span></p></li><li><p><span style="color: rgb(0, 0, 0);">2. Decreases Hb O2-binding</span><span style="color: rgb(0, 0, 0);"><br></span><span style="color: rgb(0, 0, 0);">affinity</span></p></li></ul></li></ul></li></ul><p>Makes hemoglobin’s binding ability harder (keeps subunits in T state)</p>](https://knowt-user-attachments.s3.amazonaws.com/118e6eab-150c-4705-ad65-ef86f2bedcbf.png)
2,3-BPG is a neg. allosteric effector of Hb
T-state: lower affinity for O2
-BPG makes O2 binding challenging not impossible when released hemoglobin able to go to R state
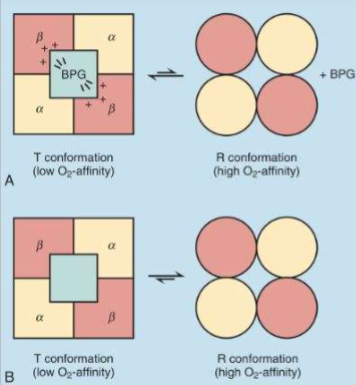
Role of 2,3-BPG
I. Important physiological regulator of Hb O2 binding
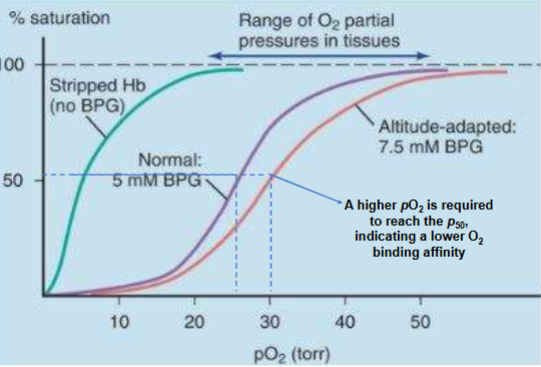
A. [2,3-BPG] increases in hypoxic conditions (what causes hypoxia/conditions of low [O2]; occurs due to lung diseases, severe anemia, & being at high altitude)
B. Does not affect Hb oxygenation in lungs
C. Does enhance Hb unloading of O2 in body tissues (decreases Hb O2 affinity; this shifts Hb oxygen-binding curve to right)
In body tissue bpg tells hemoglobin to let go of O2 (increases O2 in body)
at high alt. bpg goes up to get more O2 release
Curve with BPG
-normal levels (purple)
-O2 binding curve right shifted (hemoglobin not binding tight to O2, more bpg present)-red
-when remove bpg O2 binds with great affinity (looks like myo. curve)- green
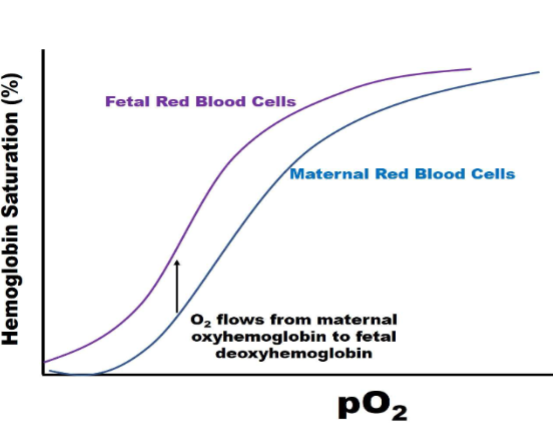
2,3-BPG &HbF
Fetal hemoglobin higher affinity for binding O2
I. Binds less tightly to HbF than HbA
A. Reduces HbF O2 binding affinity to a lesser extent (has a higher O2 binding affinity than HbA)
1. HbF p50 = 20 torr
2. Facilitates transfer of O2 from maternal blood to fetal blood in placental
capillaries
Fetal Hb curve
-left sift (higher affinity for O2)-purple
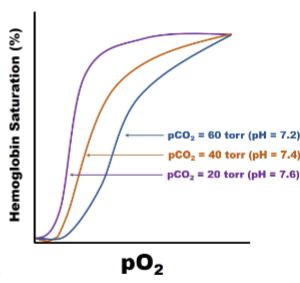
Bohr Effect pt1
I. Acidification of blood reduces O2 binding affinity of Hb, resulting in release of bound O2
A. Caused by CO2 formation, which drives carbonic acid buffer system reaction to produce H+
B. Caused by lactic acid production during anaerobic
metabolism
Acidifying bc too much CO2 in system (disrupts Co2+ H2O reverse HCO3- +H+)
right shifted (O2 affinity going down)
Bohr Effect pt1
I. O2 binding to Hb causes release of H+ from Hb
A. At lower pH, excess H+ will rebind to Hb, inducing release of O2
B. Hb + O2 ⇄ Hb * O2 + nH^+
O2 favors hemoglobins acidic affinity
II. Overall consequence is Hb most easily releases O2 in actively metabolizing tissues where it is most needed
A. Hb also serves as a blood buffer
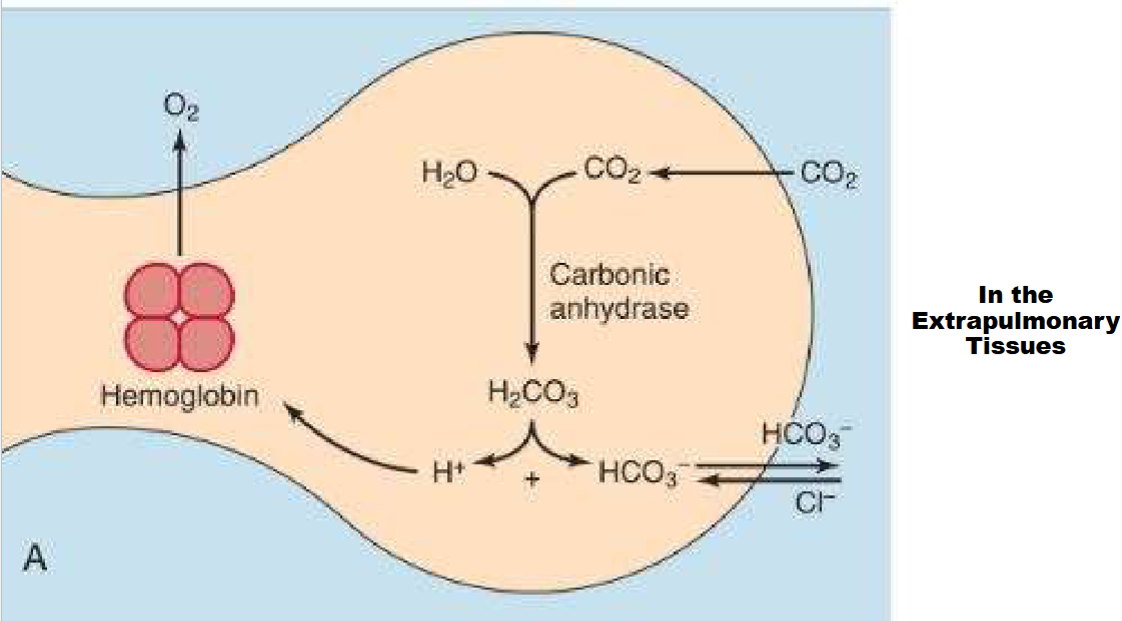
Hb Role in Co2 Transport pt1
CO2 has higher H20 solubility than O2
SOme transported dissolved in blood plasma
CO2 bind to terminal amino groups of Hb chains to form Carbaminohemoglobin (reduces Hb O2 binding affinity, causing easy release of O2 into actively metabolizing tissues (where the CO2 is
produced)
Hb Role in Co2 Transport pt2
Isohydric transport
A. Bohr effect on Hb & carbonic acid buffering system lead to reversible conversion of CO2 into HCO3-
1. Major transport form of CO2 in the blood (~ 80% transported this way)
In the Extrapulmonary tissues (main way CO2 moves in blodd as bicarbonate)
Be able to explain process in pic
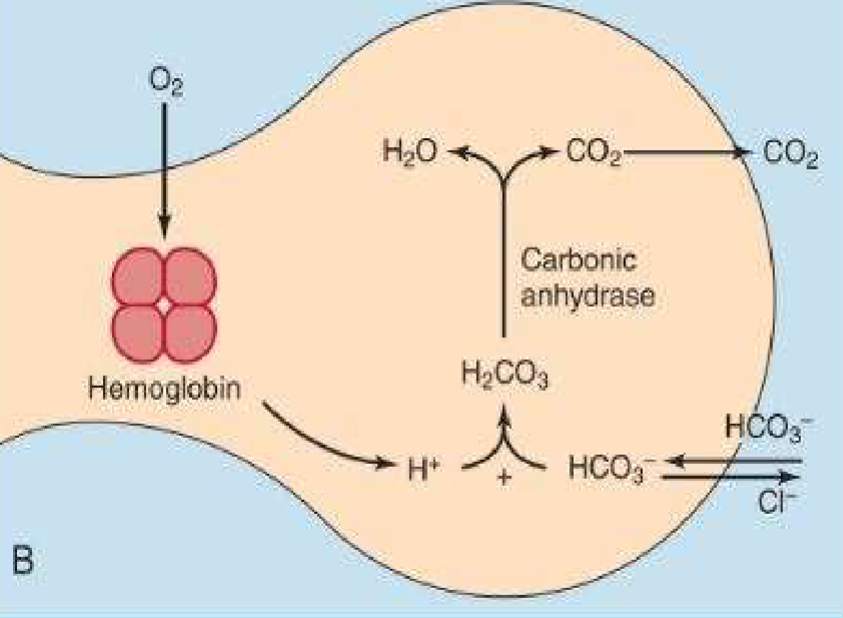
Transport of Blood Gases (In the Lungs)
Be able to explain process in pic
-favors reverse rxn transport of blood gases
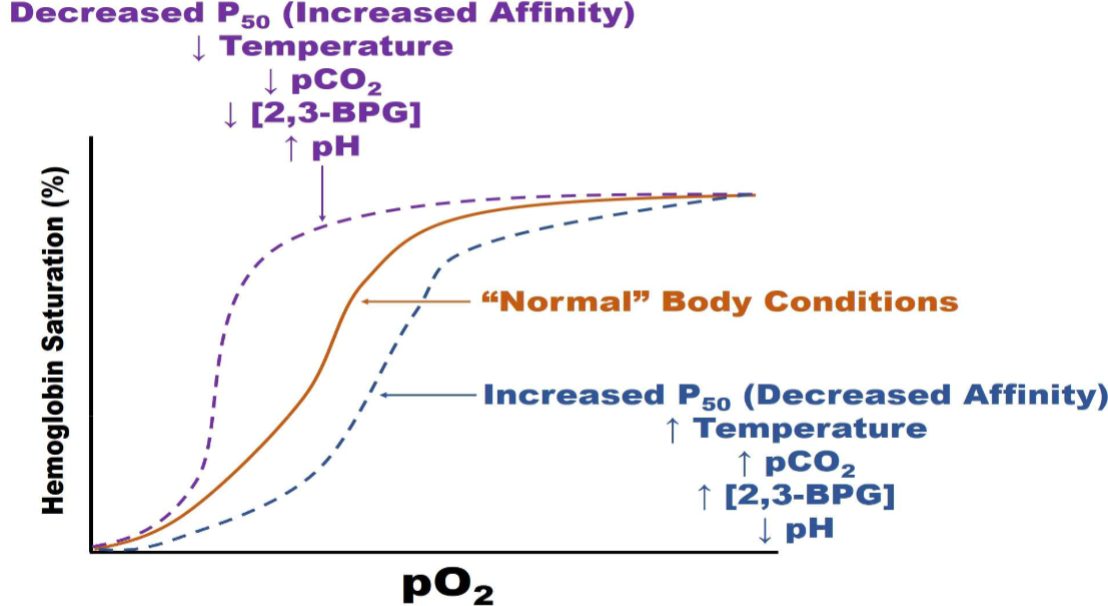
summary of slides
Orange brown- sigmoidlal curve normal ph (7.35), norm. range of bpg
Purple - Left shift, bind more tightly to O2
what left shifts curve?: Affinity: decrease in pco2 and decrease temp., increase ph
Blue line (right shift)
What right shift: increase body temp, increase partial presure of cabon dioxide, decrease in
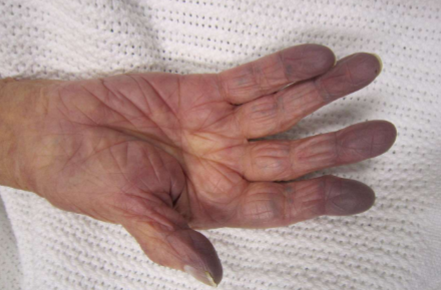
Cyanosis
Bluish/purplish discoloration of skin, lips, &
mucous membranes resulting from
inadequate oxygenation of blood
Carbon Monoxide Poisioning Pt 1
Carbon monoxide (CO) is formed from incomplete combustion of organic compounds (e.g., present incigarettes smoke, Hookah smoke, & car exhaust)
II. Reversibly binds to ferrous iron in Hb & Mb with 200x greater affinity than O2
A. Competitively antagonizes O2 binding
B. Produces carboxyhemoglobin, which is
incapable of carrying O2
Once Bound its really hard to break off
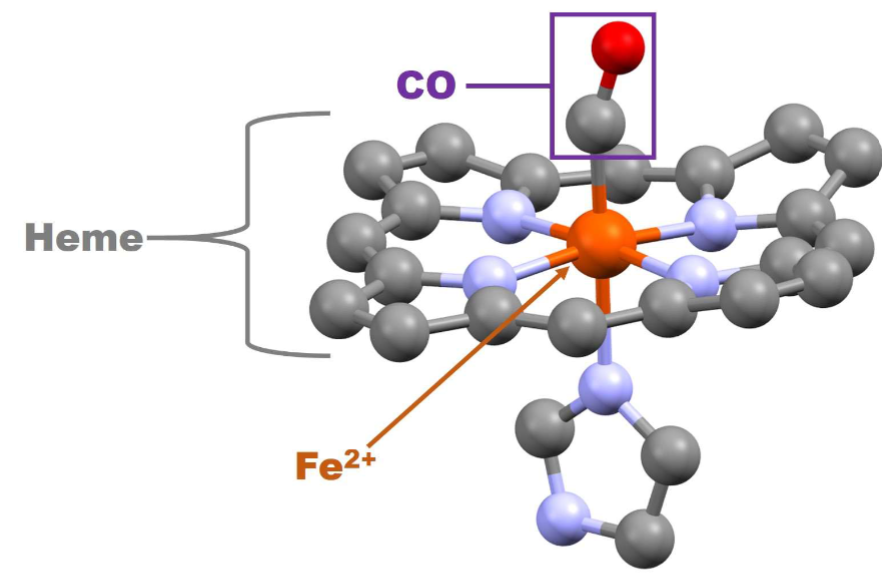
Carbon Monoxide Posioning pt 2
I. Symptoms include appearance of cherry red skin & nails, headache, dizziness, nausea, confusion, fainting, & death (at 70% Hb saturation with CO)
II. Treated with 100% O2
Microcytic Hypochromic Anemia
I. Condition caused by impaired Hb synthesis
A. Can result from Fe deficiency due to poor nutrition &/or chronic blood loss
B. Can result from vitamin B6 deficiency (needed for heme synthesis)
C. Can result from thalassemias (genetic diseases in which Hb synthesis is impaired)
II. In this condition, RBCs are depleted of Hb, taking on a small appearance (microcytic) & pale color (hypochromic)
Methemoglobinemia Pt1
I. Caused by the oxidation of heme ferrous iron to ferric iron (produces methemoglobin; metHb)
A. MetHb does not bind or carry O2 (causes brown color of dried blood)
II. Caused by exposure to certain compounds & drugs (e.g., aniline dyes, aromatic nitro compounds, nitrites, & some local anesthetics)
III. Symptoms: dizziness, headache, anxiety, dyspnea, & death (occurs when patients achieve 70% concentration of metHb)
-emia ( refers to something w/ blood)
Methemoglobinemia Pt2
I. RBCs express an enzyme called methemoglobin reductase
A. Uses NADH to reduce metHb back to Hb
1. Deficiency in enzyme causes congenital methemoglobinemia
II. Treated with methylene blue
A. Is enzymatically reduced to a product that can reduce heme iron back to ferrous state42
Hemoglobinopathies
def: Inherited defects in globin genes (causes structural & functional changes in Hb)
Sickle Cell Anemia pt 1 (Hemoglobinopathies)
Sickle cell anemia
A. Occurs predominately in black populations
(~ 0.25% African Americans; ~ 4% West Africans)B. Caused by a single AA change in the HbA
beta-chain (Glu6 → Val), forming HbS1. Disease only occurs in individuals that
are homozygous for this mutation (if hetero dont have it)
Sickle Cell Anemia pt 2 (Hemoglobinopathies)
Mutation causes solubility of deoxyHb to dramatically decrease
1. In the capillaries of body (where pO2 is low & [deoxyHb] is high), HbS molecules polymerize inside RBCs
i. Causes distortion of RBCs’ shape (produces classical sickle shape)
1) Sickled cells get stuck in capillaries, compromising blood flow
a) O2 deprivation causes necrosis & lysis of RBCs & severe pain
![<ul><li><p><span style="color: rgb(0, 0, 0);">Mutation causes solubility of deoxyHb to dramatically decrease</span></p><ul><li><p><span style="color: rgb(0, 0, 0);">1. In the capillaries of body (where pO2 is low & [deoxyHb] is high), HbS molecules polymerize inside RBCs</span></p><ul><li><p><span style="color: rgb(0, 0, 0);">i. Causes distortion of RBCs’ shape (produces classical <strong>sickle shape</strong>)</span></p><ul><li><p><span style="color: rgb(0, 0, 0);">1) Sickled cells get stuck in capillaries, compromising blood flow </span></p><ul><li><p><span style="color: rgb(0, 0, 0);">a) O2 deprivation causes necrosis & lysis of RBCs & severe pain</span><span style="color: rgb(0, 0, 0);"><br></span></p></li></ul></li></ul></li></ul></li></ul></li></ul><p><span style="color: rgb(0, 0, 0);"><br></span></p>](https://knowt-user-attachments.s3.amazonaws.com/b1fdeb34-db89-4058-984f-7556f6cf18fd.png)
Sickle Cell Anemia pt 3 (Hemoglobinopathies)
Individuals that are heterozygous for the sickle cell trait have about 65% HbA & 35% HbS
1. Their RBCs do not sickle under ordinary body conditions; they’re healthy
2. Individuals display natural malaria resistance
i. W/ HbS it either reduces malarial parasite growth or lead to early destruction of parasitized cells
ii. Bc of this, sickle cell trait has been maintained with populations living within malarious regions
a) This is an example of heterozygote advantage
Thalassemias (Hemoglobinopathies) pt1
A. Group of inherited syndromes caused by imbalances in the synthesis of alpha & beta globin chains
1. alpha-thalassemia (alpha-chain synthesis is impaired)
2. beta-thalassemia (beta-chain synthesis is impaired)
B. Heterozygosity for a thalassemia mutation causes thalassemia minor (benign condition); homozygosity for a thalassemia mutation causes thalassemia major (severe disease)
Thalassemias (Hemoglobinopathies) pt2
A. Symptoms include anemia, Fe overload, infection, bone deformities (due to enlarged bone marrow), enlarged spleen, slow growth rate, & heart problems
B. Treatments depend on type & severity of condition
1. Include blood transfusions, bone marrow transplants, & iron chelators