Caps lecture notes- Midterm 2
1/208
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
209 Terms
composition of blood (L17)
Most of the blood is made of plasma (mostly water), then RBCs and then only a little WBCs and platelets
functions of blood (L17)
Transport and protection:
Supply oxygen to tissues (haemoglobin)
Removal of waste (e.g. carbon dioxide)
Immunological functions (e.g. WBCs)
Coagulation (stop bleeding)
Messenger functions (e.g. hormone transport)
Maintains body temp. & acid-base balance
determination of hematocrit (L17)
Hematocritt is the % of RBCs in blood
Blood volume is made up of:
Plasma 55-60% → water, proteins, nutrients, hormones etc.
Hematocrit (RBCs) 40-45%
Buffy coat (WBCs and platelets) <1%
Blood volume = plasma volume + hematocrit
~5.5L = 3L plasma (55%) + 2.5L hematocrit (45%)
functions of blood proteins (L17)
Albumin: maintenance of oncotic pressure and transport
Lipoproteins: lipid transport
Glycoproteins:
Transferrin → Fe3+ binding
Coagulation factors: hemostasis
Immunoglobulins: immunity
Complement: immunity
structure and function of red blood cells (RBCs) (L17)
Function only in peripheral blood stream
Bind O2 for delivery to tissues
In exchanged, bind CO2 for removal from tissues
They have a unique discoid shape
Maximizes SA: ~140µm2
Important for gas exchange → the shape means hemoglobin (Hb) close to more areas of the membrane
Donut appearance under light microscope
RBCs have a lifespan of ~120 days since they get damaged when going through tight spaces like capillaries
Structure
Have a cell membrane and cell cytoplasm
The RBCs are malleable (squishy) so that multiple can flow through a venule and single RBCs can flow through capillaries
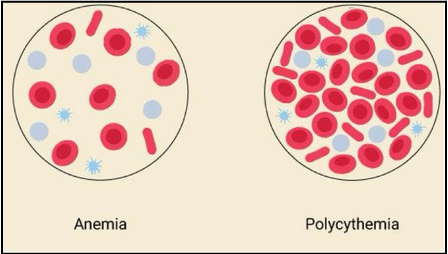
anemia and polycythemia (L17)
Anemia: reduced capacity to carry oxygen
Not always due to reduced number of RBCs
Can be caused by iron deficiency (cannot bind oxygen to heme), pernicious (B12 deficiency) or hemorrhagic
Polycythemia: too many circulating RBCs
Too viscous and can lead to blood clots and eventually strokes
how to identify the causes of anemia and polycythemia (L17)
Medical history
Physical exam
Blood tests - commonly CBC
Peripheral blood smear
Oxygen saturation
JAK2 mutation (polycythemia)
what is heme? (L17)
Heme is an iron-containing molecule
Critical component of proteins like hemoglobin
Responsible for transport of oxygen and carbon dioxide
why do we care about iron in RBCs? (L17)
Key component of hemoglobin (carries oxygen from lungs to tissues)
Iron binds to heme
Essential for bone marrow to produce new RBCs
Iron-deficiency anemia= fatigue, weakness
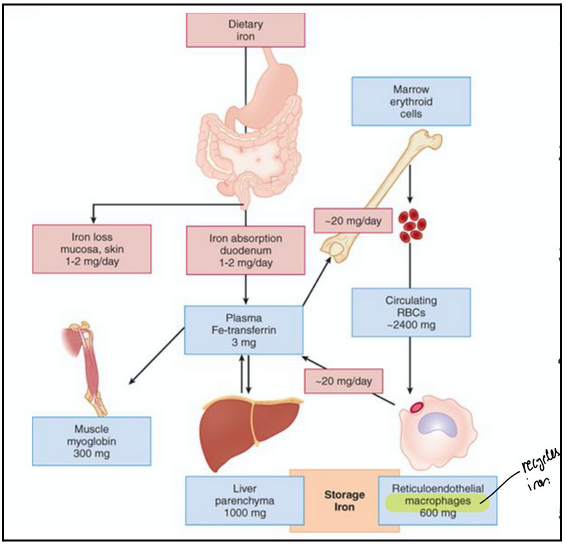
iron metabolism (L17)
Absorption: Fe2+ absorbed through intestinal mucosa (duodenum)
Oxidation: ceruplasmin (oxidative enzyme) oxidizes Fe2+ to Fe3+ (ferric)
Transport: Fe3+ binds to and is transported through blood by transferrin
Incorporation: in erythroblasts Fe3+ is reduced to Fe2+ and incorporated into heme, which is then incorporated into Hb
Storage: ferritin binds and stores excess iron (bone marrow and liver)
High transferrin levels are likely bad since the body will keep pumping transferrin if no binding occurs
hemoglobin (Hb) synthesis (L17)
Hb synthesis occurs in bone marrow, specifically, in erythoblasts and reticulocytes
Adult Hb requires two parts:
Heme (iron-containing compound; non protein part of Hb)
Globins (proteins)
Heme synthesis starts in the mitochondria → continues in the cytosol
Globins (part of adult Hb) synthesis occurs on polyribosomes in cytosol
Hb synthesis in reticulocytes (L17)
The reticulocyte is the stage of the RBC that still has some RNA (mostly ribosomal RNA) but has extruded its nucleus. So, hemoglobin synthesis continues into the reticulocyte stage, even though the nucleus is gone.
Reticulocytes still contain residual ribosomal RNA (rRNA).
This residual rRNA allows a small amount of protein synthesis (mostly hemoglobin) to continue after the cell has left the bone marrow.
Once the reticulocyte enters the bloodstream, it gradually loses all RNA.
At this point, it becomes a fully mature RBC, which cannot synthesize any new proteins because it has no nucleus and no RNA.
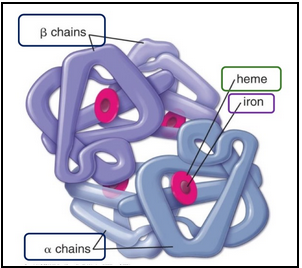
adult hemoglobin structure (L17)
It’s made of four globin chains, each bound to a heme group that contains Fe²⁺ (iron)
Most commonly 2𝛽and 2𝜶 chains → HbA
They are proteins that surround and protect heme
In adults, there are four globin types: α, β, δ, γ.
There are further A subtypes
Example: HbA1c of clinical significance
Glycosylated Hb
Meaning glucose from plasma attaches non-enzymatically to hemoglobin.
The fraction of hemoglobin that is glycated reflects the average plasma glucose over the past 2–3 months (lifespan of RBCs).
A1c < 7% is generally considered good glucose control in diabetes.
Not always an accurate measurement of diabetes
heme structure (L17)
Non-protein part (porphyrin ring)
Iron (Fe2+) inside
Iron reversibly binds O2
Transports from lungs to tissues and picks up CO2 on way back
Covalently bound to globins
In globins: iron-containing heme groups
Veins are blood due to the absorbance of light
Good to know:
CO2 doesn’t compete for Fe2+ it bidns the Hb protein chains (globins) but CO competes fiercely (binds 250x better for Fe)
Will win and cause rapid O2 starvation which is why carbon monoxide poisoning is so rapidly fatal
blood types (L17)
RBCs have different surface antigens
A+ blood is type A blood that also has Rh** surface antigen
A- blood is type A blood that does not have Rh** surface antigen
O- blood is type O blood that does not have Rh** surface antigen
Universal donor
AB+ blood is a universal acceptor
**Rh: protein on surface of RBCs → determines if a person is Rh+ or Rh-
Critical factor in blood transfusions
What would happen if type B- blood was given to a person with type A blood? (L17)
The recipient's anti-B antibodies will recognize the B antigens on the donor RBCs. This will cause aggutination (clumping) of the donor RBCs.
Note that the donor antibodies have little effect unless it’s a large transfusion because the antibodies get diluted.
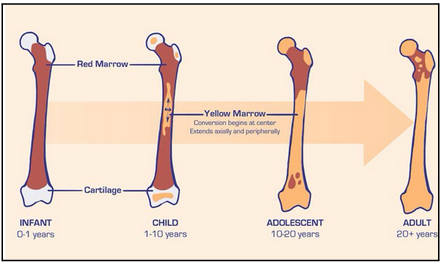
what is bone marrow? (L18)
Spongy tissue in medullary cavities of bone
Location of new blood formation
Very active tissue and has two types
red and yellow marrow
red marrow (L18)
Packed with dividing stem cells and precursors of mature blood cells
yellow marrow (L18)
Inactive bone marrow; dominated by fat cells
May be reactivated (i.e. extreme blood loss)
normal changes in location of bone marrow (L18)
In adults, only in heads of femur and humerus (long bones) and sternum, ribs, cranium, pelvis, vertebrate (flat bones)
Important to know red:yellow marrow ratio as it is and indicator of health
Typically 50:50 ratio where both exist
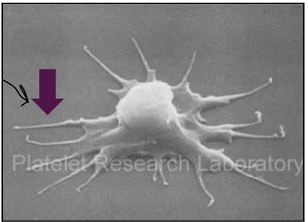
megakaryocytes and platelets (L18)
Megakaryocytes live in bone marrow
They are multi-nucleated (endomitosis without cytokinesis; up to seven duplications without cell division) and 30-150µm
Platelets are cytoplasmic fragments that break off and enter the peripheral blood stream. They function in blood clotting.
There are 1000-5000 platelets per megakayocyte
characteristics of platelets (L18)
Lifespan of around 10 days
Disc shaped with a diameter of 2-3µm
Pseudopodia allow for shape alteration and movement
No nucleus but have mitochondria, ribosomes
Granules are found in platelets:
Alpha granules: coagulation factors, adhesion molecules
Dense granules: ADP, ATP, Ca2+
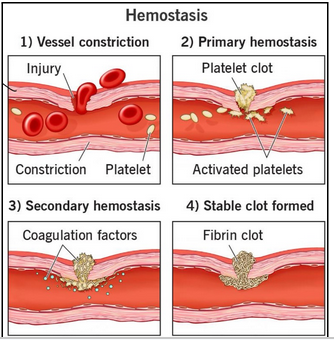
hemostasis overview (L18)
Normal hemostatic response acts to arrest bleeding following injury to vascular tissue
Four stages:
Blood vessel constricts (smooth muscle) - helps reduce immediate blood loss
Platelet clot - circulating platelets stick to damaged vessel and form a temporary platelet clot
Coagulation cascade - coagulation factors amplify clotting effects to stabilize plug
Fibrin clot - fibrin joins the party to form a solid, stable clot (during further healing, tissue replaces this)
platelet plug formation (primary wound healing) (L18)
In response to vessel wall injury, platelets adhere to the site of injury:
Von Willebrand Factor (vWF) = exposed collagen fibres in vascular wall
Activated platelets undergo conformational change (pseudopodia formation) and release alpha and dense granules → ADP and fibrinogen initiate platelet aggregation
ADP causes the release of thromboxane A2 from activated platelets → it is a potent vasoconstrictor and potentiates platelet aggregation
Results in the formation of an unstable platelet plug
Plug may be sufficient in small injuries
Plug is localized due to ADP-induced prostacyclin (released by endothelial cells; inhibits platelet activation) and NO release (inhibits adhesion, aggregation of platelets)
platelet plug formation (L18)
The overall aim of the coagulation cascade is to create a stable fibrin clot to complete the seal.
This requires thrombin production (dependent on three enzyme complexes)
Thrombin acts on fibrinogen (factor I) to promote clot formation
Important that clotting is localized which is done by a network of amplification and negative feedback loops
Extrinsic and intrinsic pathways
The intrinsic pathway is activated by factors in the blood, while the extrinsic pathway is activated by tissue factors
Both pathways cause an activation of factor X which leads to the common pathway and ends with converting fibrinogen into fibrin which forms a stable blood clot.
anticoagulation (L18)
We know clots aren’t permanent, but how do we get rid of them? → fibrinolysis
Driven by an enzyme called plasmin
Plasmin is formed from its inactive precursor plasminogen with plasminogen activators
Main two:
Tissue-tyope plasminogen activator (tPA)
Urokinase-type plasminogen activator (uPA)
Once plasmin is activated it cleaves fibrin which breaks down the clot and it is cleared from the body.
disorders of hemostasis (L18)
Platelet abnormalities
Thrombocytopenia - too few platelets; bleeding, bruising, slow clotting
Thromobocytosis - too many platelets; increased blood clot risk
Hemophilia - inherited deficiency of specific clotting factors; injury can result in uncontrolled bleeding
Type A- deficiency of factor VIII
Type B - deficiency of factor IX
Von Willebrand disease
Inherited disorder of platelet adhesion (deficiencies in factor VIII and vWF)
Injury leads to increased bleeding
Latrogenic coagulopathy
Problems with bleeding or clotting caysed by medical interventions
Eg. use of anticoagulants or antiplatelet medications
what is granulopoiesis (leukopoiesis)? (L18)
Development of white blood cells in bone marrow
Neutrophils, eosinophils and basophils
Granulocyte macrophage colony stimulating factor (GM-CSF)
Secreted by immune cells to stimulate production of more immune cells, especially granulocytes (and monocytes)
leukocytes (L18)
Leukocytes = white blood cells (WBCs)
Mobile units of immune system
Recognize and destroy or enutralize foreign materials
two types: granulocytes and agranulocytes
granulocytes (L18)
polymorphonuclear (PMNs) - specific granules in cytoplasm:
Neutrophils- phagocytose e.g. bacteria and fungi= first responders
Eosinophils- fight parasitic infections and mediate allergic reactions
Basophils- allergic responses (histamine), parasitic infections
agranulocytes (L18)
mononuclear- lack specific granules:
Monocytes- phagocytose viruses, bacteria, fungi → enter tissues and become macrophages (‘housekeepers’)
Lymphocytes- B and T cells= immune functions
complete blood count CBC (L18)
Leukocytes are least numerous blood cells in blood → ~ 1 WBC for every 700 RBCs
This is not because fewer WBCs are produced but because WBCs are only in transit while in the blood.
Never Let Monkeys Eat Bananas
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
Neutrophils are the most abundant whereas the least is basophils in the blood
neutrophils (L18)
Granulocyte= contain granules
3-5 lobed nucleus (>5 is abnormal development)
Cytoplasm filled with pale-staining granules (hence ‘neut-’)
First defenders against bacterial infections (will be elevated on CDC if infection occurs)
Phagocytosis of bacteria, release web of neutrophil extracellular traps (NETs) that contain bacteria killing chemicals
Thus, they can kill intracellularly (phagocytosis) and extracellularly (NETs)
Act as scavengers to clean up debris, e.g. old RBCs, damaged tissue

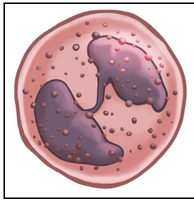
eosinophils (L18)
Granulocyte = contains granules
Bi-lobed nucleus
Cytoplasm filled with pink-staining granules (hence ‘eosino-’)
First defenders against parasites (e.g. worms)
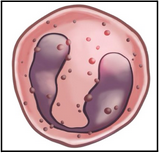
basophils (L18)
Granulocyte = contain granules
Bi-lobed nucleus
Nucleus obscured by the density of overlying granules
Cyotplasm filled with blue-purplish-staining granules (hence ‘baso-’)
Lowest cell count in CBCs often read as zero
Involved in allergic responses (contain heparin and histamine) and bacteria, fungi and viruses
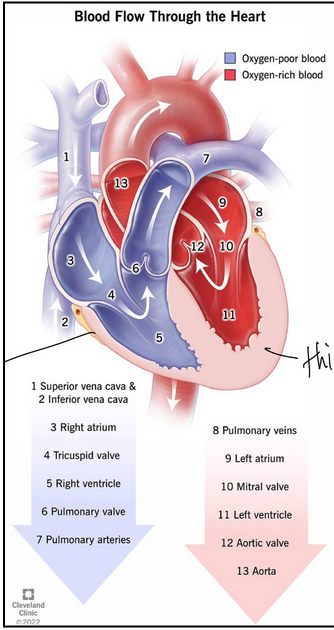
flow of blood through the heart (L19)
Deoxygenated blood returns from body via superior and inferior vena cavae
Enters the right atrium
The right atrium contracts and pushes blood through tricuspid valve
Enters the right ventricle
The right ventricle contracts pushes blood through pulmonary valve into lungs
In lungs blood picks up O2 and releases CO2
O2 rich blood returns through pulmonary veins
Enters the left atrium
The left atrium contracts and pushes blood through mitral valve
Enters the left ventricle
The left ventricle contracts pushes blood through aortic valve
Enters the aorta and can go back to the body
atrio-ventricular valves (L19)
The tricupsid and mitral (bicuspid) valves
Located between the atria and ventricles
Tricuspid
Between right atrium and right ventricle
Three cusps (anterior, septal and posterior)
At the base of each cusp anchored (chordae tendineae) to fibrous ring that surrounds orifice
Mitral (bicuspid)
Between left atrium and left ventricle
Regulates the blood flow between them
Two leaflets: anterior (aortic) and posterior (mural)
They are supported by two structures → chordae tendinae and papillary muscles
semilunar valves (L19)
Pulmonary and aortic valves
Located between ventricles and their corresponding artery
Regulate blood flow of blood leaving the heart
Pulmonary valve
Between the right ventricle and pulmonary artery
Three leaflets (anterior, left and right)
Attached to a touch, fibrous ring called annulus
Main function: allow blood flow from right ventricles to pulmonary artery and prevent backflow into right ventricle
The leaflets overlap to ensure complete closure and prevent backflow of blood into the right ventricle
papillary muscles (L19)
Small, cone-shaped muscles in ventricle
Play crucial role in valve function: contract during systole (prevents AV valves from collapsing into atria, ensure one-way flow and prevent regurgitation)
chordae tendinae (L19)
Functions with papillary muscles to prevent blood backflow
Connect the papillary muscles to AV valve leaflets
Prevents valve leaflets from being pushed back into the atria
what is the cardiac cycle? (L19)
The cardiac cycle is the complete sequence of physiological events that occur in the heart, from one heartbeat to the next
Systole = phase of chamber contraction
Diastole = phase of chamber relaxation and filling
The atria contract
The ventricles contract (AV valve closes, semilunar valve opens, blood ejected into great vessels)
Atria relax
Ventricles relax (semilunar valve closes, AV valve opens)
Note: valve are one-way = when they open, blood flows out/ when they are closed, no blood leaks back
Note: we usually associate systole with ventricular systole - period of ventricular contraction which is time between AV valve closure and semilunar valve closure
“Lub Dub” heart sounds (L19)
This sound is caused by the closing of heart valves during each cardiac cycle
Lub= also known as S1 produced by closing of AV valves (mitral and tricuspid)
Dub= also known as S2 produced by closing aortic and pulmonary valves as blood ejected from ventricles
Abnormal heart sounds occur as a result of abnormal valve movements or abnormal cardiac movements. Can also occur in healthy people.
Physiological splitting of S2:
Normal separation of the second heart sound into two components
Aortic valve closure and pulmonary valve closure are not synchronized during inspiration
Aortic valve closes slightly before pulmonic and this splits S2 into two distinct components
heart murmurs (L19)
A sound heard as a result of turbulent flow in the heart
Aortic stenosis
Normal flow across a narrowed valve
Mitral regurgitation
Across a valve which doesn’t close properly (backflow)
Ventricular septal defect
Through a hole, from a high pressure chamber to low pressure chamber
three tunics of blood vessels (L20)
Tunica intima (innermost)
Endothelium, basement membrane, connective tissue
Tunica media (most variable layer- related to functions)
Smooth muscle, elastic fibres, connective tissue
Tunica adventitia
Loose connective tissue, blood vessels, nerves
Lumens of all vessels are lined by endothelial cells but capillaries are only composed of the endothelial layer and its basement membrane — they lack the media and adventitia. Why? (L20)
to allow for easier gas exchange
thickness of arteries and veins (L20)
There is a progressive thinning of vessel walls from artery → arteriole → capillary (where gas exchange occurs), then gradual thickening again from venule → vein as blood returns to the heart.
three arteries (L20)
Large (elastic) arteries
Aorta, pulmonary arteries
Convey blood from heart to systemic circulation
High pressure vessels
Med (muscular) arteries
Most arteries
Distributing vessels
Arterioles
Start of microcirculatory bed
Resistance vessels
Arterioles still have smooth muscle in their tunica media, unlike capillaries.
That smooth muscle lets arterioles constrict or dilate, controlling:
Blood flow into capillary beds
Blood pressure (they are called the “resistance vessels” of the circulation)
three veins (L20)
Large veins eg. vena cava, femoral
Med veins: most veins (contain 70% of blood); run with arteries
Venules: receive blood from capillaries
Travel with arterioles
Venule lumens less regular in shape
Capacitance vessels (volume storage)
capillaries (L20)
Wall is one endothelial cell thick
Designed for easy and rapid exchanges between blood and tissues
Pericytes wrap around endothelial cells:
Regulate blood flow
Phagocytes
Permeability of BBB
three types of capillaries (L20)
Continuous:
Endothelial cells form a continuous, unbroken lining (tight junctions between cells)
Small gaps only at intercellular clefts where small molecules can pass
Surrounded by a complete basement membrane
Allow limited exchange — mainly small molecules like water, ions, and gases (O₂, CO₂)
Prevent loss of plasma proteins and blood cells
Found in tissues that require a tight barrier: Muscle, skin, lungsand central nervous system (CNS) → forms part of the blood-brain barrier
Fenestrated:
Endothelial cells have fenestrations (pores) in their plasma membranes.
Pores may have thin diaphragms covering them.
The basement membrane is still continuous.
Allow more rapid exchange of water and small solutes (like glucose, hormones, ions).
Still retain large proteins and cells due to intact basement membrane.
Found in tissues with high exchange or filtration: Kidneys, small intestine (villi), endocrine glands and ciliary body of eye
Sinusoid
Have an incomplete basement membrane and intercellular gap
vascular endothelium (L20)
Simple squamous epithelial cells (called epithelial cells)
Crucial for vascular functions and homeostasis
Forms a thin, continuous inner lining of all blood vessels — arteries, veins, and capillaries (~60,000 miles total in the body!).
Rests on a basement membrane.
Serves as a selective barrier and an active regulator of blood flow, clotting, and vessel health — essential for maintaining vascular homeostasis
how do veins pump blood against gravity? (L20)
Through valves
Through surrounding skeletal muscles contraction
Through tons of smooth muscle (media and adeventitia)
neural control of arteriolar diameter (L20)
SNS:
Noradrenaline- vasoconstriction- through alpha-adrenergic receptor stimulation - increases vascular resistance
Adrenaline- vasoconstriction - how is not known
PNS: insignificant
hormonal control of arteriolar diameter (L20)
Hormones (released from endocrine glands) can cause vasoconstriction and vasodialation by interacting with receptors on smooth muscle cells lining arteriolar walls
Constrictors: angiotensin II, arginine vasopressin (AVP)
Dilator: atrial natriuretic peptide (ANP)
tissue metabolites of arteriolar diameter (L20)
Produced by active tissues (e.g. increased activity during exercise) - act as vasodilators
E.g. adenosine- potent vasodilator, CO2- vasodilation, lactate (by-product of anaerobic metabolism)- vasodilation, oxygen- affects release of vasoactive substances
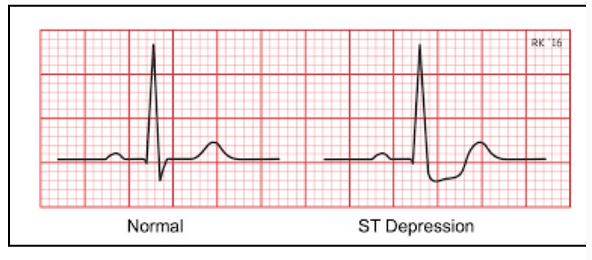
angina (chest pain) (L20)
Angina occurs when ischemia (decreased oxygen) in the heart activates afferent pain pathways, sending signals to the brain that are perceived as chest pain. If blood flow is not restored, this can progress to a heart attack (myocardial infarction).
ECG will show ST depression
cardiac output (L21)
Cardiac output= the volume of blood ejected from the heart every minute (units: mL/min)
Heart rate= number of heart beats per minute
Stroke volume= volume of blood ejected from left ventricle with each sytosolic contraction
CO= heart rate (HR) x stroke volume (SV)
regulation of cardiac output (parasympathetic) (L21)
Increased parasympathetic activity
Negative feedback on heart rate and positive feedback on CO
HR decreases (slower heartbeat)
Filling time increases → more blood fills the ventricles between beats
Stroke volume (SV) can increase due to the Frank–Starling mechanism (the heart pumps what it receives — more filling = stronger contraction)
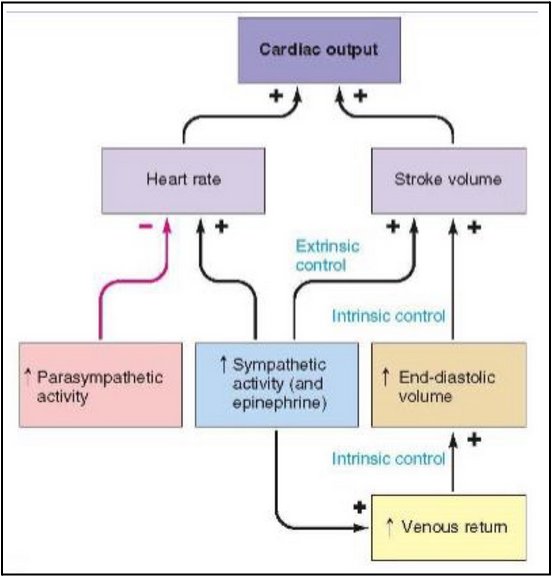
regulation of cardiac output (sympathetic) (L21)
Increased sympathetic activity
Epinephrine
Positive feedback on HR, SV, venous control
Increased end diastolic volume
Positive feedback on stroke volume
Positive feedback on CO
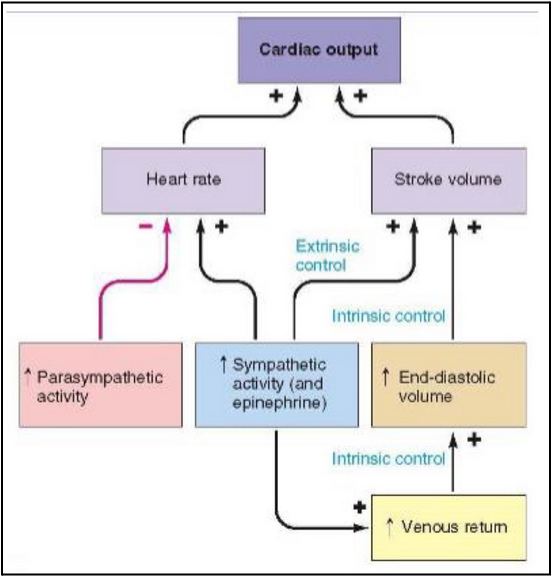
regulation of cardiac output (stroke volume) (L21)
Sympathetic nerves (releasing NE)
Acting on 𝛽1 receptors on cardiac muscle cells → increased intracellular calcium and increased stroke volume
In ventricle this means increased calcium entry into the myocytes from outside cell and calcium release from intracellular stores → promote contraction of ventricle
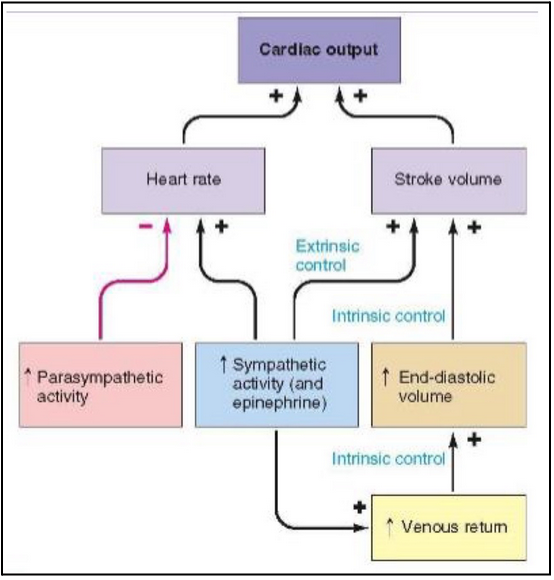
Frank Starling law of the heart (L21)
States that the strength of contraction is related to initial length of cardiac muscle fibres
The more stretched the muscle is initially, the stronger the contraction
So what? Why is this important for the heart?
The initial length of the heart muscle before contraction is equivalent to how filled the heart is at the end of diastole, or the end of the diastolic volume
The strength of contraction is equivalent to the stroke volume during systole
Bottom line: if you increase ventricular end diastolic volume (or preload) you increase the stroke volume (within reason, the heart will pump what it receives)

pressure and resistance (L21)
CO= cardiac output
Blood pressure= CO x resistanceCO= volume in vessels
Resistance = diameter of vessels (systemic vascular resistance or SVR)
So, what determines SVR?
Resistance is determined by the radius of the blood vessels
Resistance = 1/r4
Inversely proportional to the fourth power of the vessel’s radius
This means a small change in radius has a significant impact on resistance, because it is raised to the fourth power
E.g. if a blood vessels constricts to half of its original radius, the resistance to flow will increase 16 times
what causes vasoconstriction (ie. increased SVR)? (L21)
Exposure to cold
Stress
Certain medications (e.g. decongestants, migraine medications, stimulants)
Raynaud’s phenomenon: spasms in response to cold, stress, emotional upset
Smoking, coffee, salty foods
what causes vasodilation (ie. decreased SVR)? (L21)
Exercise (muscles require more oxygen and nutrients, vasodilation increases blood flow)
Low oxygen levels (hypoxia)
Increased body temp. (helps release heat through skin, aiding in cooling)
Inflammation: deliver more oxygen and nutrients
vasoactive hormones (L21)
Constrictors:
Angiotensin II
Arginine vasopressin
Dilator:
Atrial natriuretic peptide (ANP)
afterload (L21)
The pressure the ventricle must generate in order to eject cardiac output
Primarily determined by resistance in arteries
Chronic high arterial BP is an example of high afterload
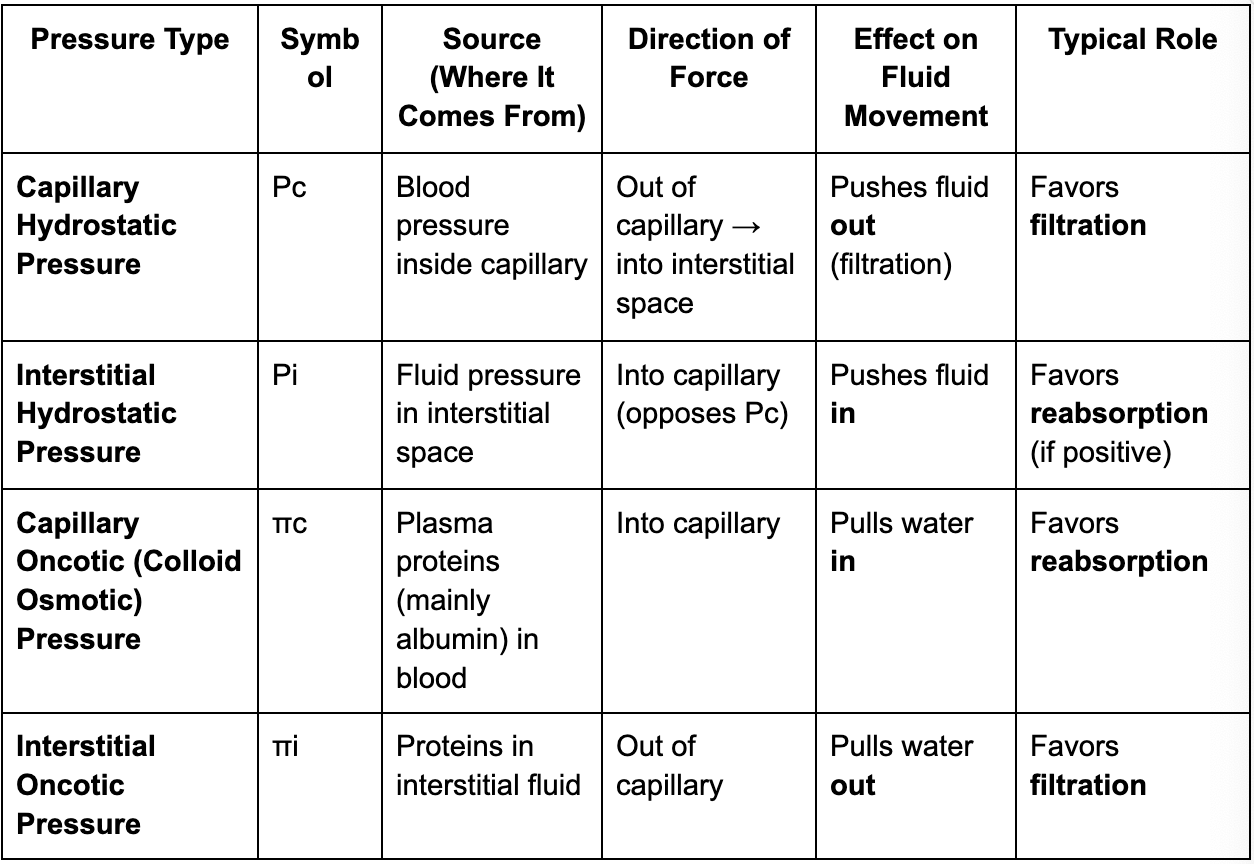
capillary hydrostatic pressure (L21)
pressure exerted by blood within a capillary, driving fluid out of the vessel and into surrounding tissues
capillary oncotic pressure (L21)
pressure exerted by proteins in the blood plasma that draws water from the interstitial space back into capillaries —> opposes hydrostatic pressure
no net movement (L21)
state where no overall movement of fluid, solutes across capillary wall, either in or out of the blood. Typically occurs when driving forces are balanced or opposed → equilibrium.
net filtration (L21)
process where fluid is pushed out of capillaries into surrounding tissues. Occurs due to differences between hydrostatic pressure (pressure of fluid w/in capillary) & osmotic pressure (pressure exerted by proteins in blood). Occurs when capillary hydrostatic pressure> blood colloid osmotic pressure. In most capillaries, more fluid is filtered out than is reabsorbed, leading to a net filtration
interstitial oncotic pressure (L21)
Interstitial oncotic pressure is the osmotic pressure created by proteins in the interstitial fluid (the fluid surrounding cells in tissues).
pulls water out of capillaries and into the interstitial fluid.
net reabsorption (L21)
overall movement of fluid from interstitial space back into capillaries. Driven by difference in osmotic and hydrostatic pressures, with osmostic pressure favouring the movement of fluid back into capillaries due to higher [protein] in blood.
table comparison of pressure types (L21)
Starling forces adjustment after hemorrhage (L21)
Overall goal: Restore intravascular (blood) volume after blood loss.
Fluid moves from interstitial space → into capillaries (net reabsorption).
After hemorrahage Starling forces shift to favour the movement of fluid from the interstitial space into the capillaries to restore blood volume
Initial and most significant change is a sharp drop in capillary hydrostatic pressure, which reduces the force of pushing fluid out of the capillaries
Hemorrhage → ↓ blood volume → ↓ capillary blood pressure.
This reduces the outward filtration force that normally pushes fluid out.
Result: Less filtration / more reabsorption into capillaries.
Followed by a slower, but critical process where proteins move from the interstitium back into the plasma, increasing capillary oncotic pressure and further promoting fluid reabsoprtion.
As plasma volume falls, plasma proteins become more concentrated.
Over time, proteins also move from interstitial fluid → plasma, further raising capillary oncotic pressure.
This increases the inward osmotic pull, enhancing reabsorption of interstitial fluid.
blood pressure (L22)
Blood pressure (BP)= pressure inside blood vessels or heart chambers relative to atmospheric pressureHow does your brain know what your BP is and what does it do about it?
How does your brain know what your BP is and what does it do about it? (L22)
Afferent nerves in medulla receive messages from baroreceptors (BP sensors) and send message back through efferent vessels to blood pressure controllers (baroreceptors)
BP cuff (L22)
Inflate cuff around upper arm to stop blood flow, then slowly release air while listening for blood flow sounds through brachial artery
Systolic pressure recorded first when first sound heard, then diastolic pressure recorded when sound disappears
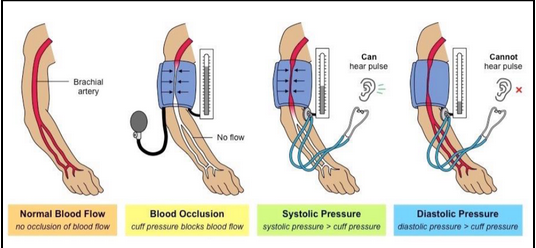
What and where are baroreceptors? (L22)
Stretch sensitive nerve endings that detect BP
Increased BP means increased nerve impulses from baroreceptors, which tells brain to lower BP by slowing heart rate and dilating blood vessels
carotid sinus and aortic arch (L22)
Carotid sinus: in common carotid artery
(afferent nerve= glossopharyngeal nerve)
Aortic arch: in arch of aorta
(afferent nerve= vagus nerve)
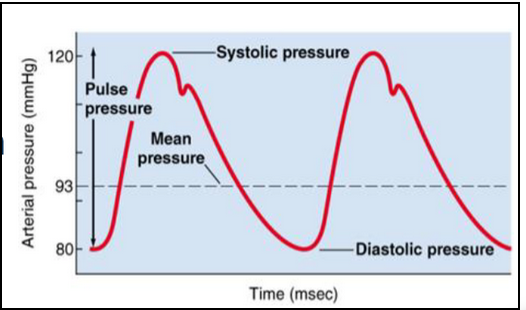
mean arterial pressure (MAP) (L22)
MAP related to systolic and diastolic pressures through a formula
MAP= DBP +⅓ (SBP-DBP)
Common way is to add diastolic to one-third of pulse pressure (= difference between diastolic and systolic)
Why care?
MAP represents average pressure in person’s arteries, indicating overall blood circulation and organ perfusion
High MAP: increased risk of cardiovascular disease
Low MAP: not enough oxygen to organs= shock, organ damage
Restoring BP after acute rise in arterial pressure (L22)
What can cause this? → stroke or hypertensive emergencies
BP≥180/120 → can lead to stroke, heart attack, kidney failure
Needs rapid interventions
IV anti-hypertensive medications that aim to reduce BP slowly (if not done slowly → headache, chest pain, shortness of breath, confusion)
Restoring BP after acute fall in arterial pressure (L22)
Can be caused by hemorrhage, heart failure, cardiac event
Treatment depends on cause:
If hemorrhage then stop bleeding and restore blood volume with fluid resuscitation and potentially blood transfusion
If heart failure or cardiac event, focus on optimizing cardiac output with medications like vasopressors to increase BP
why do we breathe? (L23)
Gas exchange
Route for water loss and heat elimination
Acid-base balance (altering amount of H+)
Speech, singing and smell
Defends against inhaled foreign matter (alveolar macrophages)
conducting structures (L23)
Nasal cavity
Nasopharynx, oropharynx, larynx
Trachea
Bronchi
Bronchioles
These all function to warm and humidify air and to remove foreign particles
respiratory structures (L23)
Respiratory bronchioles
Pulmonary alveoli
Alveolar ducts
Alveolar sacs
Alveoli
These all function in gas exchange
common micro-anatomical plan lungs (L23)
Mucosa: respiratory epithelium, basement membrane
Submucosa: loose connective tissue containing seromucous glands
Adventitia: outer connective tissue layer, binds airways to adjacent structures (so lungs aren’t floating freely)
respiratory epithelium (L23)
Functions in protection (pathogens), mucociliary clearance, humidity and warming, gas exchange and immune defense
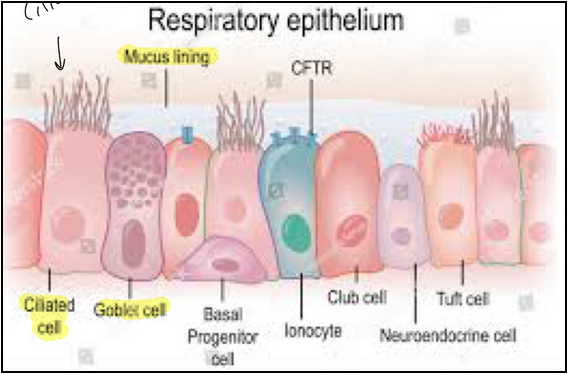
airway lining fluid (L23)
Composed of two layers
Mucus layer: gel-like substance, 97% water, 3% solid
Pericilliary layer: low viscous fluid
Catch foreign debris
Note: mucus is produced by both goblet cells and seromucous glands
Muco-ciliary escalator (L23)
Cilia provide coordinated sweeping motion that moves mucus and entrapped foreign materials to the larynx for expulsion
Tracheobronchial tree (L23)
Trachea splits into two bronchi
Airway further divides around 23 times finally reaching 150-250 million alveoli in each lung
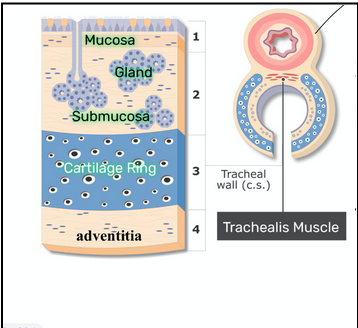
Trachea (L23)
Contains
Mucosa: respiratory epithelium
Submucosa: seromucous glands
Cartilage: 16-20 rings, maintains patency of tube (prevents collapse)
Adventitia: loose connective tissue
Note: the cartilage is not continuous so that the trachealis muscle can stretch and allow for swallowing
main bronchi (L23)
Contains
Mucosa: respiratory epithelium
Submucosa: seromucous glands
Cartilage: 16-20 rings, maintains patency of tube (prevents collapse)
Adventitia: loose connective tissue
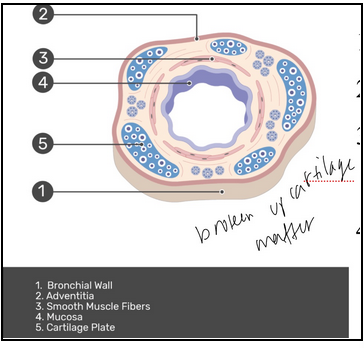
intrapulmonary bronchi (L23)
Lots of branching
As they become smaller:
Decreased cartilage
Epithelial height reduced (need only one cell layer for easy gas exchange)
Smooth muscle becomes prominent
bronchioles (L23)
Very small conducting airways
No cartilage
No submucosal glands
Epithelial lining transitions:
Respiratory → simple columnar → simple cuboidal
Goblet cells replaced by club cells
club cells (L23)
Dome-shaped
Make up 80% of cells lining bronchioles
Lung protective functions:
Surfactant (reduce surface tension)
Respiratory distress syndrome in babies born without because lungs not developed enough
Treated with surfactant replacement
Inflammation control
Enzymes to break down mucus
Antimicrobial lysozymes
respiratory bronchioles (L23)
Transition point in respiratory system
From air conduction to gas exchange
Initial segments are ciliated cuboidal
Club cells persist in initial segments and become dominant in distal segments
Epithelial height is reduced
turbinates (L23)
Also known as nasal conchae
Tiny structures in the nose (bony projections)
Help regulate airflow and roles in warming, humidifying and filtering air
pulmonary alveoli (L23)
These are the terminal air spaces
Primary site for gas exchange
Air brought into very close proximity to blood
Structure:
Septae
Network of capillaries
Alveolar macrophages
Type 1 pneumocytes:
Squamous cells
Large SA for gas exchange
Cover 95% of alveolar surface
Type 2 pneumocytes:
Cuboidal cells
More numerous but only cover 5% of SA
Secrete surfactant
blood air barrier (L23)
Minimal thickness
Type 1 pneumocytes: thin
Capillary endothelium: thin
Allows for rapid gas exchange
Diffusion of oxygen from alveoli to capillaries and diffusion of carbon dioxide out of capillaries to alveoli. Across the epithelium
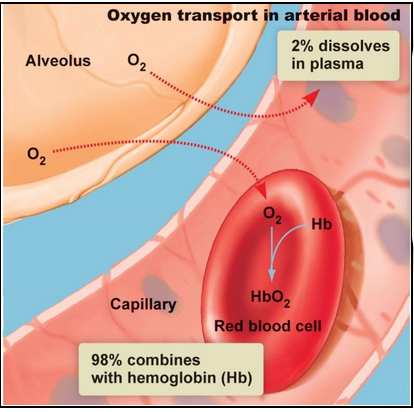
Oxygen transport in the blood (L23)
Hemoglobin (protein) contains iron which readily binds to oxygen
When this occurs it is called oxyhemoglobin
Hemoglobin allows for vast majority (98%) of oxygen to be transported (faster than if it was dissolved in plasma)
Hemoglobin facilitates oxygen transport
Hemoglobin binds with oxygen in lungs
Has 4 binding sites
Oxygen binds and becomes oxyhemoglobin
The partial pressure difference (higher in alveoli compared to blood) drives oxygen from the alveoli to blood