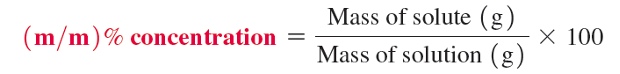CHMY 121N - Chapter 8 and 9
1/131
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
132 Terms
change of state
The change of a substance from one state of matter (gas, liquid, or solid) to another
melting point
The temperature at which the liquid phase is in equilibrium with the solid phase
boiling point
The temperature at which the gas phase is in equilibrium with the liquid phase
intermolecular forces (IMFs)
forces that act between molecules or discrete atoms and hold them close to one another
aka van der Waals forces
intramolecular forces
Forces within atoms of the same molecule
london dispersion force
temporary attractive forces between molecules created by electron movement
weak strength
get stronger as the size of the molecule increases
aka induced dipole force
do london dispersion forces have a short-lived or long-lived polarity?
short lived
at any given instant there may be more electrons at one end of a molecule than at the other
do all molecules experience london dispersion force, or only some?
all molecules
The larger the molecular weight and surface area, the greater the _______ of a molecule
temporary polarization
dipole-dipole forces
occurs between the positive end of one polar molecule and the negative end of another polar molecule
gets stronger for more polar molecules
weak strength
do molecules that contain polar covalent bonds have a net polarity?
yes, they could.
Dipole-Dipole forces are stronger the more _____ the molecule.
polar
ion dipole force
Interaction between a fully charged ion and a polar molecule
hydrogen bond
occurs between molecules with O-H, N-H, and/or F-H bonds
gets stronger for more polar molecules
moderate strength
hydrogen bond donor
H atom with a covalent bond with N, O, or F (most electronegative elements) can provide a hydrogen atom
hydrogen bond acceptor
Another molecule with a lone pair of electrons on N, O, or F (most electronegative elements) accepts hydrogen atoms
can non-polar molecules form hydrogen bonds?
no
In liquids and solids, the stronger the intermolecular forces, the ______ the melting and boiling points.
higher
what are the 3 intermolecular forces from strongest to weakest?
Hydrogen bonds > dipole-dipole > london dispersion
ideal gas
A gas that obeys all the assumptions of the kinetic-molecular theory
non-ideal gas
don’t completely follow these assumptions because of intermolecular forces
Kinetic-Molecular Theory of Gases
Gas particles move randomly and don’t attract or stick to each other.
The particles are tiny compared to the space between them.
Hotter gases (higher temperature) have faster-moving particles.
When gas particles collide with each other or the container, they bounce off without losing energy.
Pressure (P)
the force per unit area pushing against a surface
what is the equation for pressure?
pressure = force / area
what is the SI unit for pressure?
pascal (Pa)
how do you get kPa?
multiply pascal by 1000
what are common units of pressure?
torr or millimeters of mercury (mmHg)
atmospheric pressure
measures the amount of pressure that gases in our atmosphere puts on one square inch
what makes up 1 atm?
14.7 lbs/in2 (psi)
what is the conversion factor for atm?
1 atm = 760 mmHg = 760 torr
gas laws
A series of laws that predict the influence of pressure (P), volume (V), and temperature (T) on any gas or mixture of gases
directly proportional
if when one variable increases, so does the other variable.
inversely proportional
if when one variable increases, the other decreases.
are area and force directly or inversely proportional?
directly
are volume and pressure directly or inversely proportional?
inversely
Boyle’s Law
P1V1 = P2V2
What is the proportionality between temperature and volume?
direct
Charles’ Law
V1 / T1 = V2 / T2
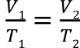
is temperature in kelvin or celcius?
kelvin (°C + 273)
What is the proportionality between temperature and volume?
direct
Gay-Lussac’s Law
P1 / T1 = P2 / T2
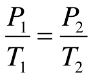
combined gas law
P1V1 / T1 = P2V2 / T2
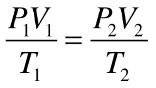
Avogadro’s Law
V1 / n1 = V2 / n2
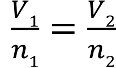
what does n represent?
moles
Standard Temperature and Pressure (STP)
0 °C (273 K) and 1 atm (760 mmHg)
Standard Molar Volume
22.4 L per 1 mol of any gas
Ideal Gas Law
PV = nRT
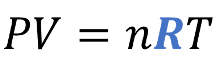
when should you use the combined gas law?
when the properties of a gas are changing, but the amount of a gas stays the same.
when should you use the ideal gas law?
when we know 3 of 4 gas variables (pressure, volume, temperature, amount) and the gas isn’t changing
The higher the molar mass, the _____ the boiling points (due to London dispersion forces).
higher
partial pressure
The contribution of a given gas in a mixture to the total pressure mixtures of gases
does each particle in a gas act independently?
yes, so the chemical identity of its neighbors is irrelevant.
do mixture of gases behave the same as pure gases?
yes, and they obey the same laws
what does the pressure exerted by each gas depend on?
the frequency of collisions of its molecules with the walls of the container.
Dalton’s Law
Ptotal = Pgas 1 + Pgas 2 + Pgas 3
atmospheric pressure
the sum of the partial pressures of all the gases in it
Viscosity
The measure of a liquid’s resistance to flowing (moving).
when does viscosity increase?
when intermolecular forces increase
surface tension
the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
what is surface tension caused by?
by the stronger inward pull on surface molecules compared to those inside the liquid.
crystalline solids
solids in which the atoms, molecules, or ions are rigidly held in an ordered arrangement.
ionic solids
particles are ions
composed of alternating positive and negative ions in 3D arrangement
held together by ionic bonds
ex: Na(s) + ½ Cl2(g) → NaCl(s)
properties of ionic solids
brittle and hard
high melting point
molecular solids
made of molecules
held together by intermolecular forces
ex: ice, wax, dry ice

properties of molecular solids
soft
low to moderate melting points
covalent networks
individual atoms held together by covalent bonds in giant 3D arrays
one huge molecule
ex: a diamond

properties of covalent networks
very hard
very high melting point
metallic solids
individual metal atoms held together by metallic bonds
valence electrons are delocalized
forming a “sea” of electrons that can move freely through the structure
this is what makes metals conductive

properties of metallic solids
lustrous
can be soft (Na) or hard (Ti)
high melting point
amorphous solids
particles are randomly arranged
no long-range structure
ex: glasses, tar, some plastics
properties of amorphous solids
noncrystalline
no sharp melting point
able to flow, though may be very slow
curved edges when shattered
vapor pressure
the partial pressure of vapor molecules in equilibrium with a liquid
What happens to liquid molecules in a closed container?
Some evaporate, but random motion brings some back into the liquid.
What is dynamic equilibrium in a closed container?
Evaporation = condensation → vapor concentration stays constant.
What affects vapor pressure?
Temperature and the liquid’s intermolecular forces.
How do intermolecular forces affect vapor pressure?
Stronger forces → fewer molecules escape → lower vapor pressure.
How does temperature affect vapor pressure?
Higher temperature → more energy → higher vapor pressure.
normal boiling point
the temperature where boiling occurs, at a pressure of exactly 1 atm
Heat of Fusion (Hfus)
The quantity of heat required to completely melt one gram of a substance once it has reached its melting point
Heat of Vaporization (Hvap)
The quantity of heat needed to completely vaporize a liquid at its boiling point
equation for melting point
heat (cal or J) = mass (g) x heat of fusion (cal or J/g)
grams cancel out
equation for boiling point
heat (cal or J) = mass (g) x heat of vaporization (cal or J/g)
grams cancel out
equation for heat (with no phase change)
Q = m × ΔT × C
q = heat (cal)
m = mass (g)
ΔT = change in temp (in Celsius)
c = specific heat (cal / (g*celcius))
homogenous mixture
the mixing is uniform and has the same composition throughout
solute
A substance that is dissolved in a solvent
solvent
The substance in which another substance (the solute) is dissolved
When one liquid is dissolved in another, the minor component is usually considered the _____ and the major component is the ______.
solute, solvent

what does “like dissolves like” mean?
Compounds with similar intermolecular forces tend to dissolve other compounds with similar intermolecular forces.
what kind of solutes do polar solvents (like water) dissolve?
polar and ionic solutes
what kind of solutes do nonpolar solvents (like oil) dissolve?
nonpolar solutes
solubility
The maximum amount of a substance that will dissolve in a given amount of solvent at a specified temperature
what does a saturated solution contain?
the maximum amount of dissolved solute at equilibrium
what is a miscible substance?
completely soluble in any proportion
cannot form saturated solutions with each other
supersaturated solution
occur when a solution contains even more solute than the saturated solution
solutions that were not saturated at high temperatures become supersaturated when they cool
these solutions are unstable
when are solids more soluble?
at high temperatures
when are gases least soluble?
at high temperatures
Henry’s Law
The solubility of a gas is directly proportional to the partial pressure of the gas if the temperature is held constant
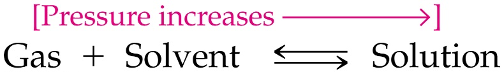
An ______ in pressure causes ____ gas molecules to enter solution until equilibrium is restored between the dissolved and un-dissolved gas
increase, more
percent concentration
expresses the amount of solute in 100 amounts of total solution
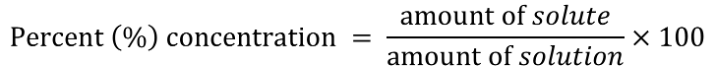
mass/mass percent concentration (m/m)%
for solid solutions