INNATE IMMUNE RESPONSE
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
characteristics of innate immunity (5)
primitive (spread across species)
unlearned, instinctive response
slow response
does not depend on immune recognition therefore has no long lasting memory
integrates w adaptive immune response
what is innate immunity composed of (3)
physical and chemical barriers
phagocytic cells: neutrophils, macrophages, dendritic cells
blood proteins: complement, acute phase
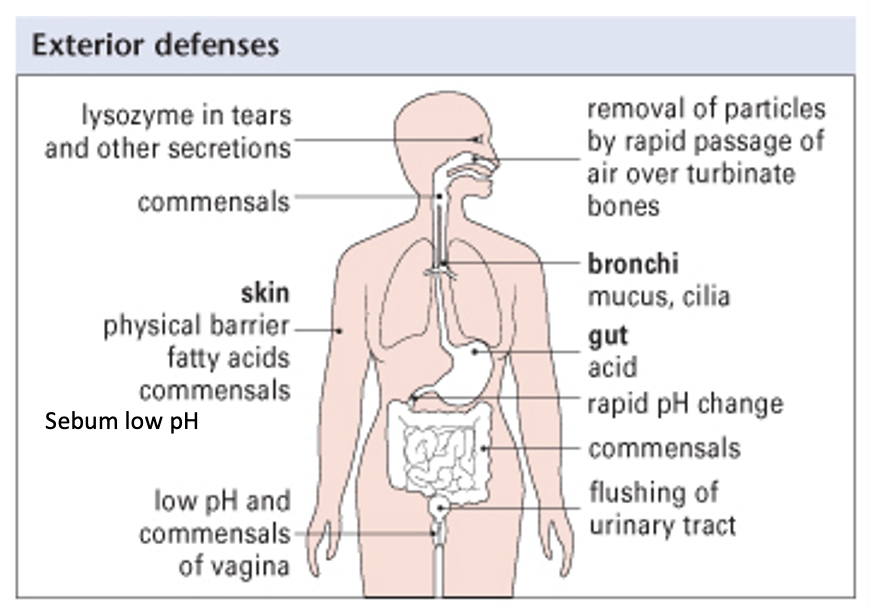
what is innate immunity composed of: physical barriers (3)
skin
mucus
cilia
what is innate immunity composed of: chemical barriers (3)
lysozyme in tears
low vaginal pH
HCl in stomach
general exterior defenses
define inflammation
a series of reactions that brings cells and molecules of the immune system to sites of infection or damage
when does an inflammatory response occur
when physical barriers are breached
process of inflammatory response (7)
Stop bleeding (coagulation)
Acute inflammation (leukocyte recruitment - macrophages and dendritic cells live in tissues)
Kill pathogens, neutralise toxins, limit pathogen spread
Clear pathogens/dead cells
Proliferation of cells to repair damage
Remove blood clot – remodel extracellular matrix
Re-establish normal structure/function of tissue
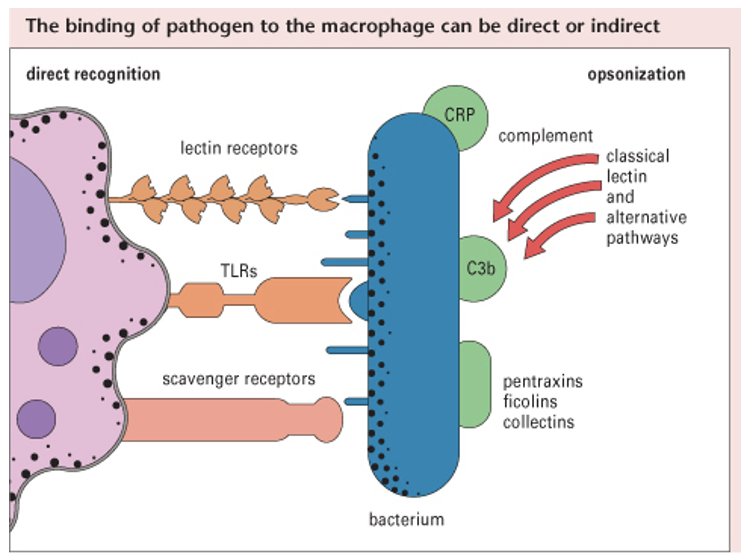
how innate immune cells sense foreign molecules/ antigens (4)
in blood: monocytes and neutrophils
in tissues: macrophages and dendritic cells (initially recognise non-self)
Pathogen Associated Molecular Patterns (PAMPs): on microbes i.e. non-self
Pattern Recognition Receptors (PRRs): on self cells (mainly on APCs + neutrophils + monocytes)
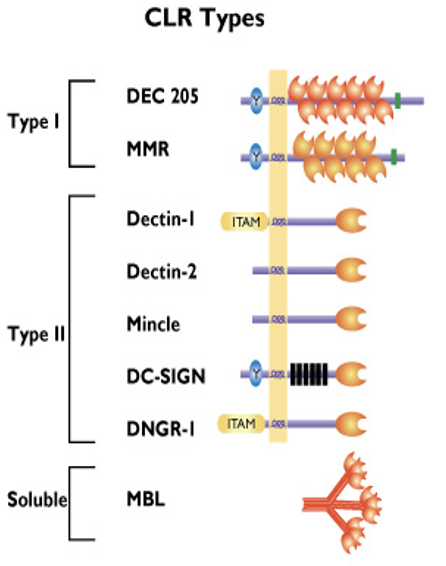
how innate immune cells sense foreign molecules/ antigens: C-Type Lectin receptors Type I
have several carbohydrate recognition domains (CRD)
DEC205 (CD205): recognise apoptotic and necrotic human cells (clean up mechanism)
Macrophage Mannose Receptor (MMR/ CD206): recognises terminal mannose, N-acetylglucosamine and fucose residues on glycans attached to proteins on surface of some microorganisms (particularly fungi and bacteria)
ONLY ON NON-SELF
how innate immune cells sense foreign molecules/ antigens: C-Type Lectin receptors
expressed by macrophages, monocytes, neutrophils and dendritic cells
bind to non-self carbohydrates in a Ca2+ dependent manner
receptors have a carbohydrate recognition domain (CRD)
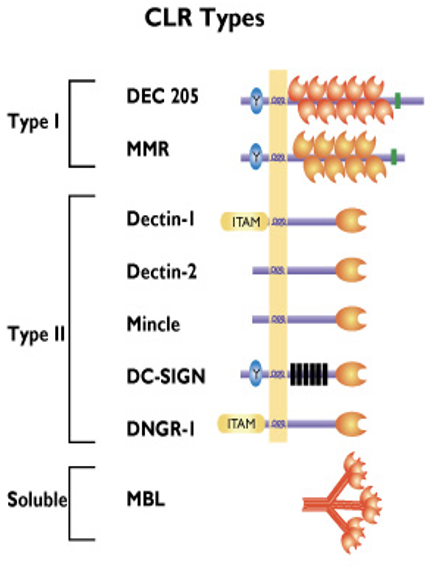
how innate immune cells sense foreign molecules/ antigens: C-Type Lectin receptors Type II
only have one carbohydrate recognition domain (CRD)
Dectin-1: binds beta-glucans that are glucose polymers found in the cell walls of fungi
Dectin-2: binds mannans (mannose-type carbohydrate) mainly on fungi
Mincle: binds mycobacteria, fungi and damage associated molecular patterns (DAMPs) released by dying human cells
DC-SIGN: recognition of several viruses e.g. HIV-1, HCV, dengue virus, Ebola and other microbes of the Leishmania and Candida species
DNGR-1: binds damaged or dead human cells via exposed actin filaments (DAMP)
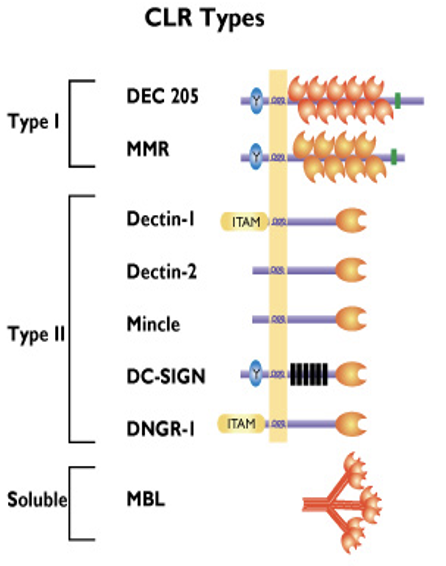
how innate immune cells sense foreign molecules/ antigens: C-Type Lectin receptors: Type III
soluble receptor
Mannose Binding Lectin (MBL): binds to repetitive mannose and/ or N-acetylglucosamine residues on microorganisms, leading to opsonization and activation of the lectin complement pathway
MBL has a crucial role in innate immunity against yeast/ fungi by enhanced complement activation and enhanced uptake by polymorphonuclear cells
MBL also interacts w carbohydrates on surface of HIV-1
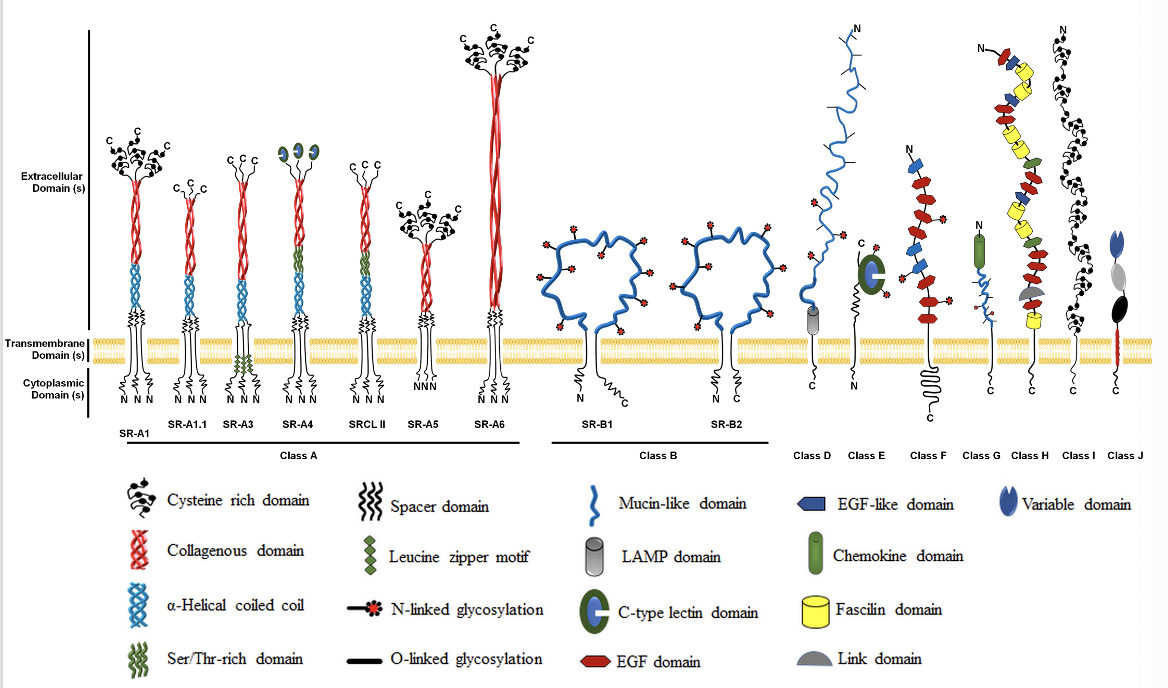
how innate immune cells sense foreign molecules/ antigens: Scavenger receptors (SRs)
mainly recognise lipids/ lipoproteins
expressed by macrophages, dendritic cells
superfamily of membrane bound receptors
bind to variety of ligands incl. host proteins and pathogens, particularly bacterial cell wall components (lipids/ lipoproteins) of Gram -ve and Gram +ve bacteria
also functions in homeostasis where SR binds and internalises lipid containing molecules e.g. modified LDL and oxLDL (oxidised LDL) from plasma
can lead to atherosclerosis if dysregulated; when oxLDL binds to macrophages it can drive atherosclerosis too
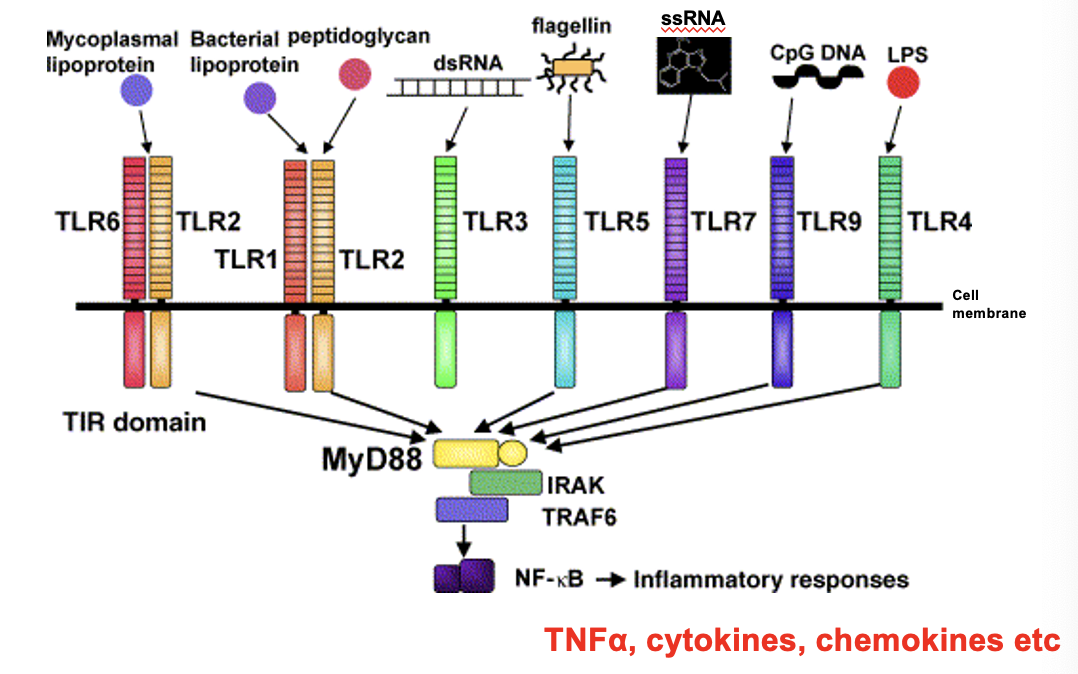
how innate immune cells sense foreign molecules/ antigens: Toll-Like receptors
recognise a variety of PAMPs expressed by microbes as well as damaged cells
different TLRs bind to Gram +ve and Gram -ve bacteria
when TLR binds to PAMPs, it is activated and causes intracellular signalling which triggers an inflammatory response
proinflammatory cytokines are transcribed (NFκB is a transcription factor), signalling cascade then activates macrophages
e.g. lipoproteins and peptidoglycans are part of Gram +ve bacterial cell walls which will bind w TLR1 and TLR2
e.g. TLR4 will bind to LPS (lipopolysaccharides) within Gram -ve cell wall
ssRNA is from viruses
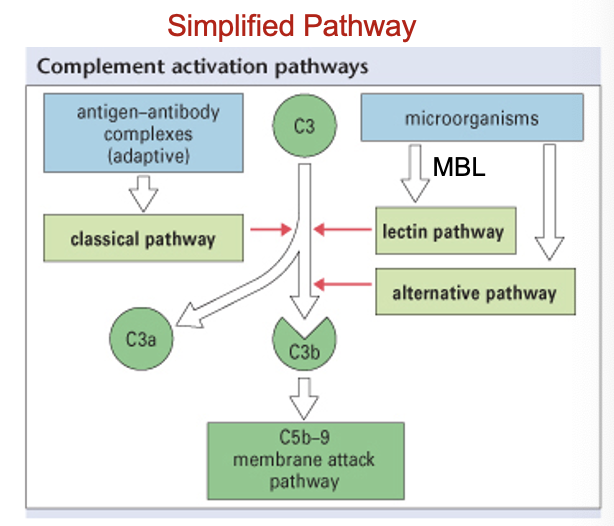
inflammation: complement (C’)
complements are a group of ≈ 20 serum proteins that need to be ‘activated’ to be functional and are secreted by the liver
activated by an immune response - normally circulates around body in an inactivated form
C’ have three different activation pathways
once C’ is activated, it causes several subsequent events to be continually activated i.e. it causes an immunological cascade
inflammation: complement (C’): activation pathways (3)
classical - activated when Ab binds to microbe
alternative - activated when C’ binds to microbe
lectin - activated when mannose binding lectin (MBL) binds to microbe
MBL is C type Lectin receptor Type III - soluble
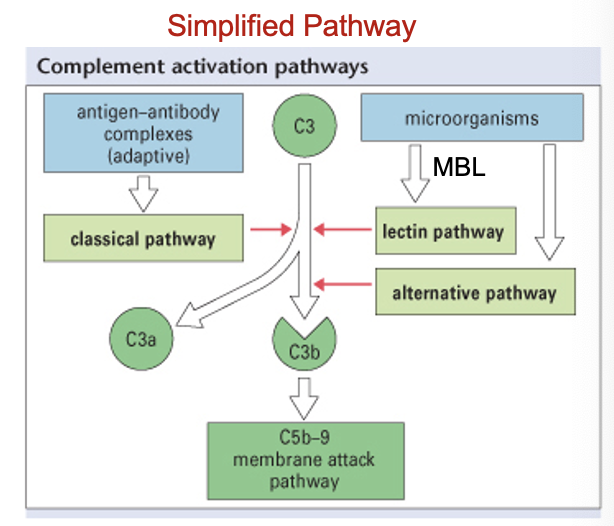
C3a and C5a; C3b; C5-C9 (all formed during complement activation)
C3a and C5a are released
both are chemoattractants which recruit leukocytes to site of inflammation
C3b gets inserted into surface of microbe - it sticks out and coats microbe (i.e. opsonisation)
C3b also activates MAC
C’ can also kill microbes directly via Membrane Attack Complex (MAC)
when C5-C9 combine they form a pore in surface of pathogen, causing contents to spill out which kills organism in process
C3b leading to microbe destruction
C3b receptor (CD11c/ CD18) is a Pattern Recognition Receptor (PRR) expressed on surface of macrophages/ neutrophils
C3b receptor recognises and binds to C3b (opsonin) when it is bound to the surface of microbes
binding of C3b to C3b receptor causes phagocytosis and destruction of the microbe
summary of C’ functions (3)
C’ can lyse microbes directly via the Membrane Attack Complex (MAC)
can cause chemotaxis via C3a and C5a (chemoattractants)
C3b can coat microbes i.e. opsonisation - helps leukocytes attracted to site of inflammation phagocytose pathogen
process of how immune cells travel from bloodstream to the site of infection (12)
once tissue macrophage senses microbe, inflammatory response occurs - gaps between endothelial cells increase
proinflammatory cytokine released e.g. TNF alpha
TNF alpha interacts w surrounding cells, causing them to produce chemokines
results in chemokine gradient (high levels at infection site)
TNF alpha activates endothelium which expresses E-Selectin (sticky factors)
neutrophils (NP) that normally pass by endothelium interacts w E-Selectin via CD15 on cell surface
NP starts to become sticky and ‘roll’ on E-Selectin i.e. tethering
as NP rolls, it encounters chemokines attached to endothelium via GAGs
chemokines bind to chemokine receptor on NP, this activates the NP
NP binds to adhesion molecules on endothelial cell surface
chemokine gradient pulls NP in through gap between endothelial cells, NP migrate up gradient to the site of infection
NP then uses other receptors on its surface to phagocytose non-self organisms
term for migration of leukocytes to site of infection
diapedesis (type of extravasation)
define extravasation
general term for movement of cells out of a blood vessel into surrounding tissue
haematopoiesis and infection
response to infection:
increased secretion of colony stimulating factors (CSFs)
increased release of leukocytes from bone marrow - especially neutrophils
during infection neutrophils increased to 11 × 103 cells/L (normally 3-7 x 109 cells/L)
lymphocytes are mainly involved in viral infections
ESR
increased Erythrocyte Sedimentation Rate (ESR) is a test
check if infection is present or not
during infection, fibrinogen levels increased which sticks erythrocytes together - this causes them to settle faster
which cells mainly participate in phagocytosis
macrophages
dendritic cells
neutrophils
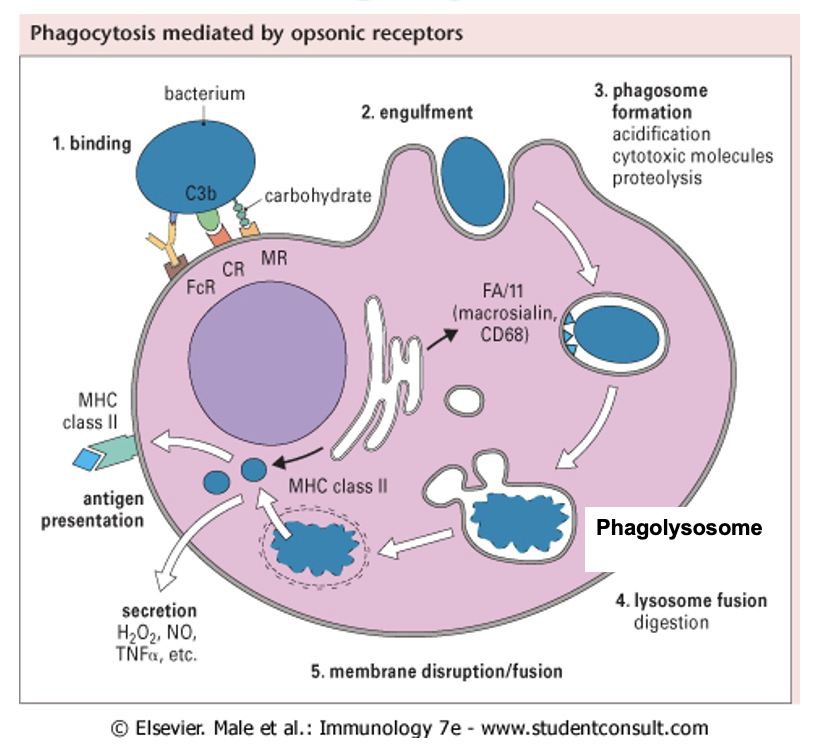
phagocytosis and antigen presentation
phagosome binds to other vacuoles (lysosomes) containing substances » phagolysosome
inside phagolysosome degrade bacterium
can reprocess other bacterium material like nucleotides
Major Histocompatibility Complex (MHC) displays the antigen on outside of immune cell - antigen presentation
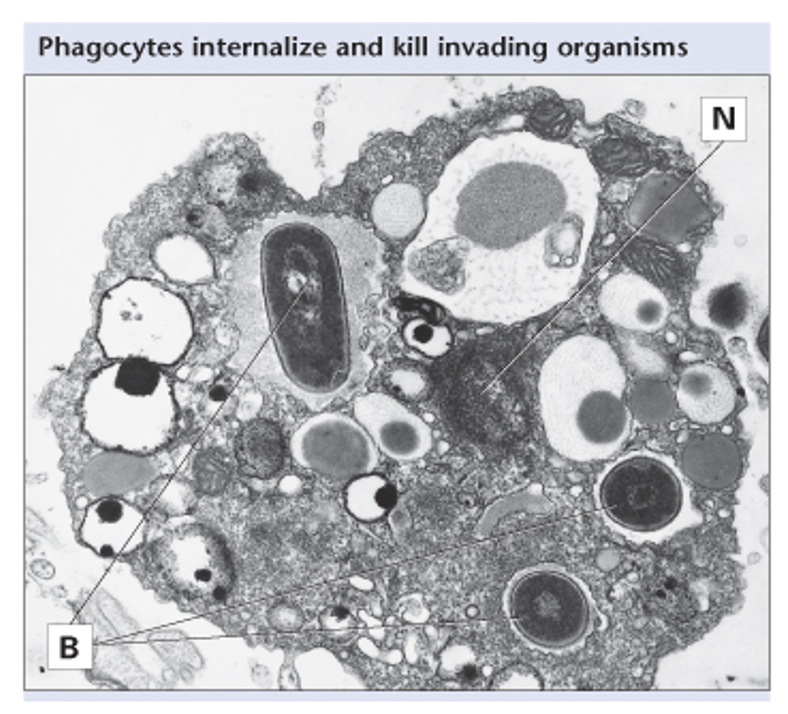
histological section of macrophage undergoing phagocytosis
N = nucleus
B= bacteria
≈ 20-30 bacteria inside macrophage
macrophages will phagocytose until they cannot anymore, then die via apoptosis
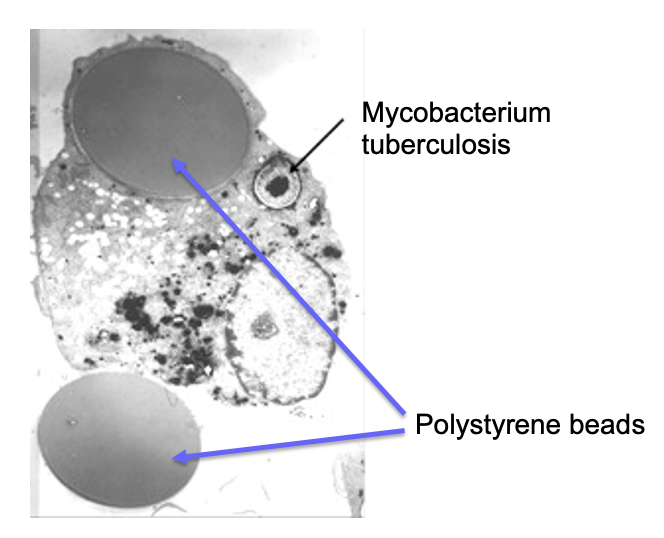
do macrophages discriminate between different foreign material?
no - as long as macrophages recognise a non-self antigen it will phagocytose the material
which cells can present antigens
macrophages
dendritic cells
B cells
NEUTROPHILS DO NOT PRESENT ANTIGENS
what process is the link between innate and adaptive immunity
antigen presentation
mechanisms of microbial killing (2)
2 killing pathways present in neutrophils and macrophages:
oxygen dependent pathway
oxygen independent pathway
^ after recognising, sensing, extravasating, phagocytosing microbe
mechanisms of microbial killing: oxygen dependent pathway (2)
Reactive Oxygen Species/ Reactive Oxygen Intermediates
superoxides e.g. O2- are converted to H2O2 which is then converted to •OH (damages DNA)
nitric oxide (NO) causes vasodilation, increasing extravasation (NO also antimicrobial)
macrophages and neutrophils + ROS production
macrophages and neutrophils produce free radicals during phagocytosis
mechanisms of microbial killing: oxygen independent pathway (3)
enzymes: lysozymes are present in tears but also secreted into phagosomes - very good at breaking down bacterial cell walls
proteins: defensins (inserted into membranes and creates pores), TNF alpha
pH: massively drops inside vacuoles