Quantisation and wave-particle duality
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
What is the concept of quantisation
The idea that values such as energy levels take only discrete values
What is the Planck constant
Has value 6.626 x 10⁻³⁴ J ∙ s
It defines the energy in a quanta
Which equation demonstrates how energy is quantised
It is important to note that energy therefore increases in steps as h is very small but not zero.

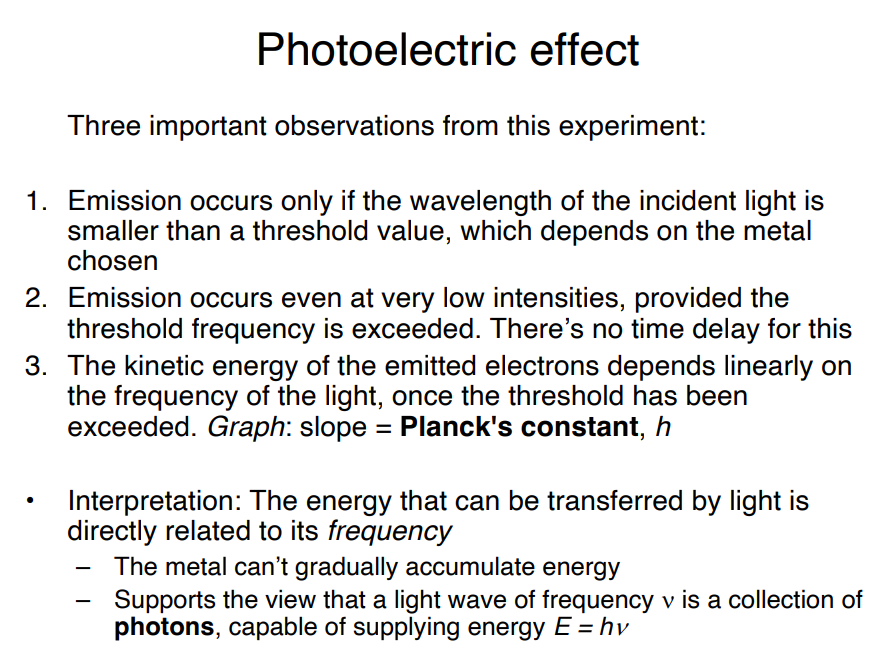
What is the photoelectric effect and its key takeaways
When high frequency/short wavelength light strikes a metal plate, electrons are emitted. Whether electrons are emitted or not and their kinetic energy is dependent on the frequency of the incident radiation. The intensity of radiation only impacts the number of emitted electrons, suggesting that light interacts with electrons in a particle-like manner i.e. as photons.
The minimum energy (determined by frequency) required to emit electrons from a metal is determined by the work function ϕ. This is measured in J or eV.
What is an eV
a unit of energy equal to the work done on an electron in accelerating it through a potential difference of one volt.
How do you calculate the velocity of an emitted electron from wavelength of incident radiation
Find the energy of the incident radiation:
Convert wavelength into frequency using λ = vc
Calculate energy using E = hv
Subtract the energy of the work function
The “leftover” energy can be used in KE = 1/2mv²