Honors Chemistry Midterm
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
What is the definition of matter?
Anything that has mass and takes up space
An example that is NOT matter
Time, sound, thoughts….etc
Steps of the Scientific Method (in order)
Ask a question/make observation
Make a hypothesis
Do an experiment
Record & analyze data
Form a conclusion
Qualitative Observation
Describe something using words
Quantitative Observation
Describe something using numbers and are usually made with instruments
What 2 words does a well written hypothesis USUALLY contain?
If/Then
What is the definition of a dependent variable?
Part of the experiment that the scientist observes or measures to see if the hypothesis is correct
What is the definition of an independent variable?
Part of the experiment that the scientist change
Chemistry is defined as the study of what?
Study of matter & the changes it undergoes
What is the definition of a physical change?
A change that does NOT produce a new substance (change in 1 or more physical properties)
What is the definition of a chemical change?
A change produces a new substance with different properties
Crushing a can is a…..
Physical change
Cutting a pizza is a…
Physical change
A silver ring tarnishing (Ag – Ag2S)
Chemical Change
Burning a log until it becomes ash is a….
Chemical Change
All phase changes (changing 1 substance from a solid-liquid-gas) are….
Physical
How are temperature and kinetic energy related?
Temperature is a measure of the average kinetic energy of particles
What happens to temperature during a phase change? What is happening to the particles during a phase change?
Temperature remains constant even though heat is being added or removed. Heat energy is being used to change the intermolecular forces
Which state(s) of matter expands to fill their container?
Gases
Which state(s) of matter take the shape of their container?
Liquids & gases
Which state of matter has the most kinetic energy? Why?
Gases - particles are moving the most/fastest
Which state of matter has the strongest intermolecular forces? Why?
Solids - particles are very close together & very attracted to neighbors
What is the definition of a compound?
Atoms of 2 or more different elements that are chemically combined
What is the definition of a mixture?
2 or more elements and/or compounds that are physically combined
What is an extensive property?
Depends on amt. of matter (Mass & Volume)
What is an intensive property?
Only depends on type of matter
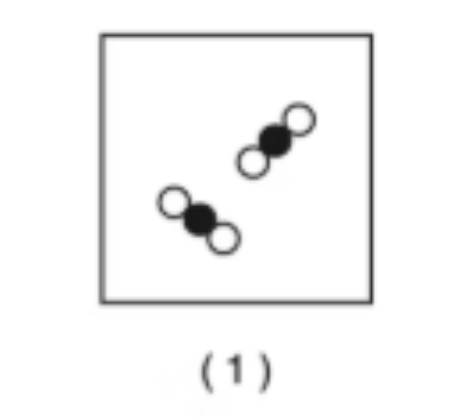
Compound
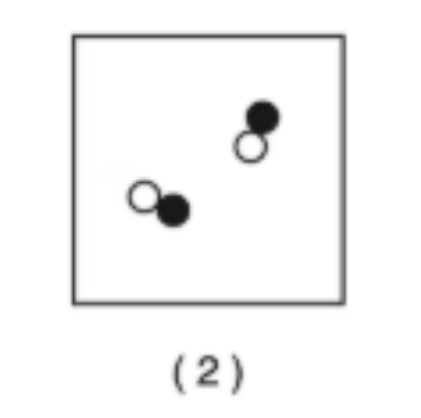
Compound
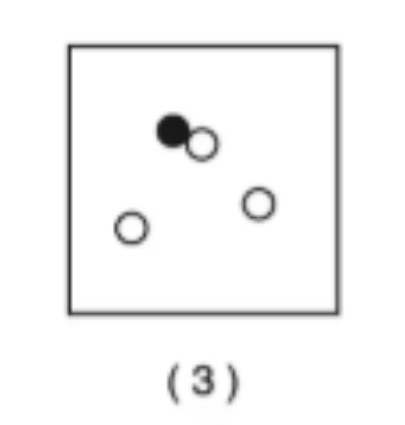
Compounds and Elements
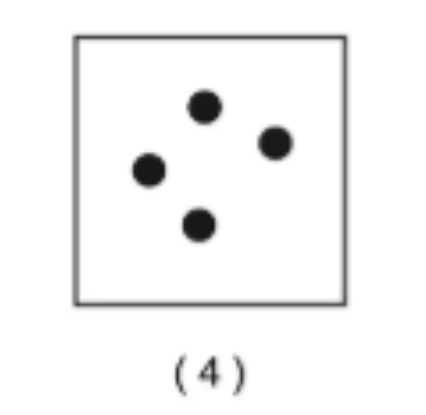
Pure element
Determine the number of atoms of each element is Ca(NO3)2
Ca = 1, N = 2, O = 6
What is the law of conservation of matter and how is it upheld in chemical equations?
Matter/atoms are neither created nor destroyed during chemical reactions. They are rearranged. This is why chemical equations must be balanced.
What is the symbol for the states of matter (solid, liquid, gas, and aqueous solution) in a chemical equation?
Solid - (s)
Liquid - (l)
Gas - (g)
Aqueous solution - (Aq)
What is the definition of a reactant? Where are they located in a chemical equation?
Starting substance in a chemical reaction / before the arrow
What is the definition of a product? Where are they located in a chemical equation?
Substance that is made/produced during a chemical reaction / after the arrow
What is accuracy?
How close a measurement is to the true value
What is precision?
How close a group of measurements are to each other
What is the equation for calculating density?
*Hint: think of 6=3/2
D=M/V
What is the equation for calculating volume?
V=M/D
What is the equation for calculating mass?
M=DxV
What is a proton?
A positively charged subatomic particle
What is an electron?
A negatively charged subatomic particle
What is a neutron?
A neutral subatomic particle
Where is the nucleus located in an atom?
The center
What 2 subatomic particles are located in the nucleus?
Protons and neutrons
Who discovered the nucleus?
Rutherford
What was the name of his experiment?
Gold Foil experiment
What is the definition of an atom?
Smallest part of an element that has its physical and chemical properties