A Level Chem 1.6 Chemical Equilibria
1/15
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
This question is about an equilibrium.
2 P(aq) + Q(aq) ⇌ R(aq) + 3 S(aq)
A 25.0 cm3 sample of a solution of P is added to a 20.0 cm3 sample of a solution of Q. The mixture is allowed to reach equilibrium.
The amounts in the equilibrium mixture are P = 0.0145 mol Q = 0.0275 mol R = 0.0115 mol S = 0.0345 mol
Explain why the amount of S increases when water is added to the equilibrium mixture. (2)
equilibrium shifts to side with most moles (1)
to oppose decrease in concentration of all reactants and products / dilution of everything (1)
This question is about the equilibrium mixture formed when A and B react.
A(aq) + 2 B(aq) ⇌ C(aq) ΔH= –32 kJ mol–1
The temperature of the equilibrium mixture is decreased. Predict the effect, if any, on the value of Kc Give a reason for your prediction. (3)
(KC) greater/increases (1)
the equilibrium shifts in the exothermic direction (1)
to oppose the temperature decrease (1)
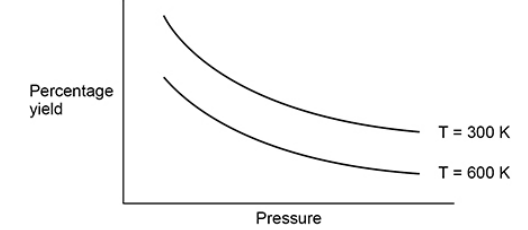
This question is about gaseous equilibria.
The diagram below shows the effect of pressure on the percentage yield of a reaction at equilibrium at two different temperatures.
Explain how the diagram shows that the forward reaction in this equilibrium is exothermic. (2)
Lower yield at higher temperature / converse argument (1)
so, equilibrium has shifted in the endothermic direction to oppose the increase in temperature (1)
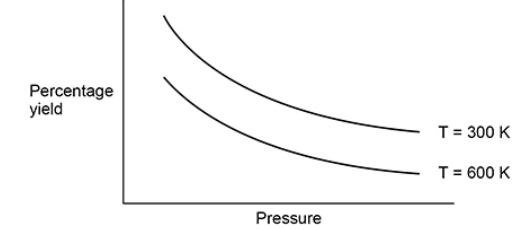
State whether the forward reaction in this equilibrium results in an increase, decrease or no change in the amount, in moles, of gas.
Explain your answer. (3)
Increase (1)
Lower yield at higher pressure (1)
So, equilibrium has shifted to the side with fewest number of moles to oppose the increase in pressure (1)
Chemists provided evidence that was used to support a ban on the use of chlorodifluoromethane as a refrigerant. Many refrigerators now use pentane as a refrigerant.
State the environmental problem that chlorodifluoromethane can cause.
Give one reason why pentane does not cause this problem.
Environmental problem _______________________________________
Reason why pentane does not cause this problem _____________________________________________ (2)
Causes ozone depletion/decomposition/damage (1)
Pentane does not have C-Cl bonds (1)
Hydrogen can be prepared on an industrial scale using the reversible reaction between methane and steam.
CH4(g) + H2O(g) ⇌ CO(g) + 3 H2(g) ΔH = +206 kJ mol−1
The reaction is done at a temperature of 800 °C and a low pressure of 300 kPa
in the presence of a nickel catalyst.
Explain, in terms of equilibrium yield and cost, why these conditions are used. (6)
Level 3 5-marks All stages are covered and the explanation of each stage is generally correct and virtually complete.
Stage 1: Temperature
1a. The reaction is endothermic (so equilibrium shifts to RHS to reduce the temperature)
1b. So, higher temperature increases the yield
1c. High temperatures are costly (so compromise temperature used)
Stage 2: Pressure
2a. More moles of gas on the right hand side, (so equilibrium shifts to RHS to increase the yield) 2b. So, lower pressure increases the yield
2c A low pressure means a low cost
Stage 3: Catalyst
3a. Catalyst has no effect on yield
3b. Adding a catalyst allows a lower temperature to be used
3c. So, this lowers the cost
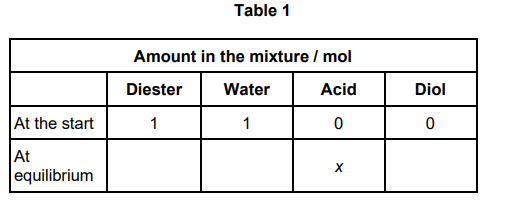
1 mol of a diester with molecular formula C7H12O4 is added to 1 mol of water in the presence of a small amount of catalyst.
The mixture is left to reach equilibrium at a constant temperature.
C7H12O4 (l) + 2 H2O(l) ⇌ 2 CH3COOH(l) + HO(CH2)3OH(l)
At equilibrium, x mol of ethanoic acid are present in the mixture.
Complete Table 1 by deducing the amounts, in terms of x, of the diester,water and diol present in the equilibrium mixture. (3)
Amount of Diester = 1- x/2 (1)
Amount of water = 1-x (1)
Amount of Diol = x/2 (1)
When one mole of ammonia is heated to a given temperature, 50 % of it dissociates and the following equilibrium is established.
NH3(g) ⇌ ½ N2(g) +3/2 H2(g)
What is the total amount, in moles, of gas in this equilibrium mixture?
A 1.5
B 2.0
C 2.5
D 3.0 (1)
A (1)
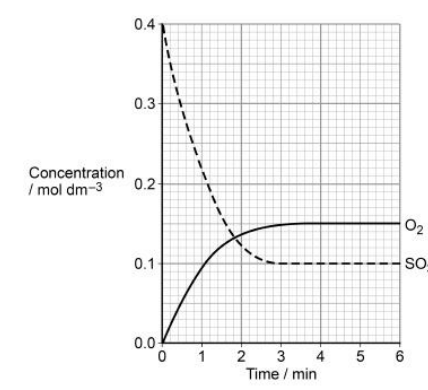
Sulfur trioxide decomposes to form sulfur dioxide and oxygen at temperature T1 according to the equilibrium shown.
2SO3(g) ⇌ 2SO2(g) + O2(g) ∆H = +196 kJ mol–1
The graph shows the concentrations of sulfur trioxide and of oxygen over a period of 6 minutes at temperature T1 State the time, to the nearest minute, when equilibrium is first established. Explain your answer.(2)
3 minutes (1)
(At equilibrium, rateforward=rateback so) concentrations (of O2 and SO3 ) remain constant (1)

Sulfur trioxide decomposes to form sulfur dioxide and oxygen at temperature T1 according to the equilibrium shown.
2SO3(g) ⇌ 2SO2(g) + O2(g) ∆H = +196 kJ mol–1
The temperature of the mixture was changed to T2 and the mixture left to establish a new equilibrium.
In the new equilibrium mixture the concentration of sulfur trioxide was found to be 0.07 mol dm–3 Deduce which of T1 and T2 is the higher temperature. Explain your deduction (2)
For T2,equilibrium has shifted to the RHS in endothermic direction (1)
Equilibrium has opposed the increase in T / Equilibrium moves to decrease the T (1)
Methanol can be manufactured in a reversible reaction as shown by the
equation.
CO(g) + 2H2(g) ⇌ CH3OH(g)
State and explain the effect of using a catalyst on the yield of methanol in
this equilibrium. (2)
no effect (on yield) (1)
increases rate / speed of both / forward and reverse reactions equally / by the same amount (1)
Methanol decomposes on heating in a reaction that is the reverse of that used in its manufacture.
CH3OH(g) ⇌ CO(g) + 2H2(g)
Use your answer from part (c) to determine the value of Kc for this
equilibrium at temperature T.
State the units for this value of Kc
ans to part (c) : 0.311 mol-2dm6 (2)
1/ 0.311= 3.22 (1)
mol2dm-6(1)
This is because Kcdecomposition = 1/ Kcmanufacture
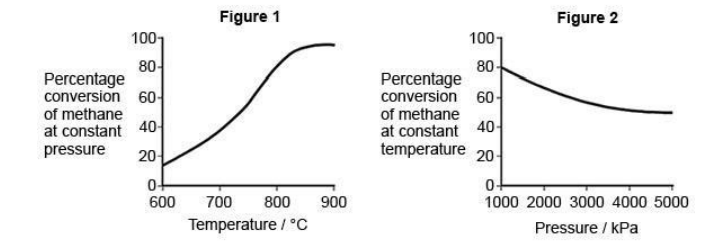
There are several stages in the industrial production of methanol from methane.
The first stage involves a gaseous equilibrium between the reactants (methane and steam), and some gaseous products. Figures 1 and 2 show the percentage conversion of methane into the gaseous products under different conditions at equilibrium.
Deduce the optimum conditions for the industrial conversion of methane and steam into the gaseous products. Explain your deductions (6)
Stage 1 – Deductions from graph
1a Yield increases as temperature increases (or converse)
1b After a certain temperature yield no longer increases
1c Yield decreases as pressure increases (or converse)
Stage 2 – Optimum temperature and explanation
2a High temperature results in high energy costs/expensive
2b (After a certain temperature) yield no longer increases therefore there is no gain in using a higher temperature
2c Optimum temperature is between 780-880oC
Stage 3 – Optimum pressure and explanation
3a Low pressure may be too slow
3b So compromise pressure required
3c Optimum pressure is 1000-2000kPa or moderate pressure used
Colourless solutions of X(aq) and Y(aq) react to form an orange solution of Z(aq) according to the following equation.
X(aq) + 2Y(aq) ⇌ Z(aq) ΔH = −20 kJ mol−1
The student prepared another equilibrium mixture in which the equilibrium concentrations of X and Z were:
X(aq) = 0.40 mol dm−3 and Z(aq) = 0.35 mol dm−3 .
For this reaction, the equilibrium constant Kc = 2.9 mol−2 dm6 . Calculate a value for the concentration of Y at equilibrium. Give your answer to the appropriate number of significant figures. (3)
answer on is on the image (3)
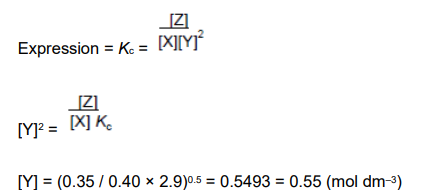
Colourless solutions of X(aq) and Y(aq) react to form an orange solution of Z(aq) according to the following equation.
X(aq) + 2Y(aq) ⇌ Z(aq) ΔH = −20 kJ mol−1
The student added a few drops of Y(aq) to the equilibrium mixture of X(aq), Y(aq) and Z(aq) in part (c). Suggest how the colour of the mixture changed. Give a reason for your answer. (3)
Darkened (1)
The equilibrium moved to the right (1)
to oppose the increased concentration of Y (1)
Colourless solutions of X(aq) and Y(aq) react to form an orange solution of Z(aq) according to the following equation.
X(aq) + 2Y(aq) ⇌ Z(aq) ΔH = −20 kJ mol−1
The student warmed the equilibrium mixture from part (c). Predict the colour change, if any, when the equilibrium mixture was warmed. (1)
The orange colour would fade (1)