Pathogenesis of viral diseases II
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
What do adapted viruses do?
Cause minimal harm
What are medical infections?
Medical infections: when viruses cause injury. Cytopathic injury facilitates virus propagation/ transmission via:
✓ Cause inflammation or physiological responses (coughing, sneezing, diarrhea), help spread the virus.
✓ Induce immune suppression → avoid viral clearance
✓ Be due to host-defense-related tissue injury
✓ Sometimes the harm is simply a by-product of viral replication (depends on host species)
What are the types of virus-induced tissue injury?
• Direct mechanisms: a direct consequence of virus replication within a cell
• Indirect mechanisms: mediated by a host immune or inflammatory response
Types of virus-cell interaction summary:
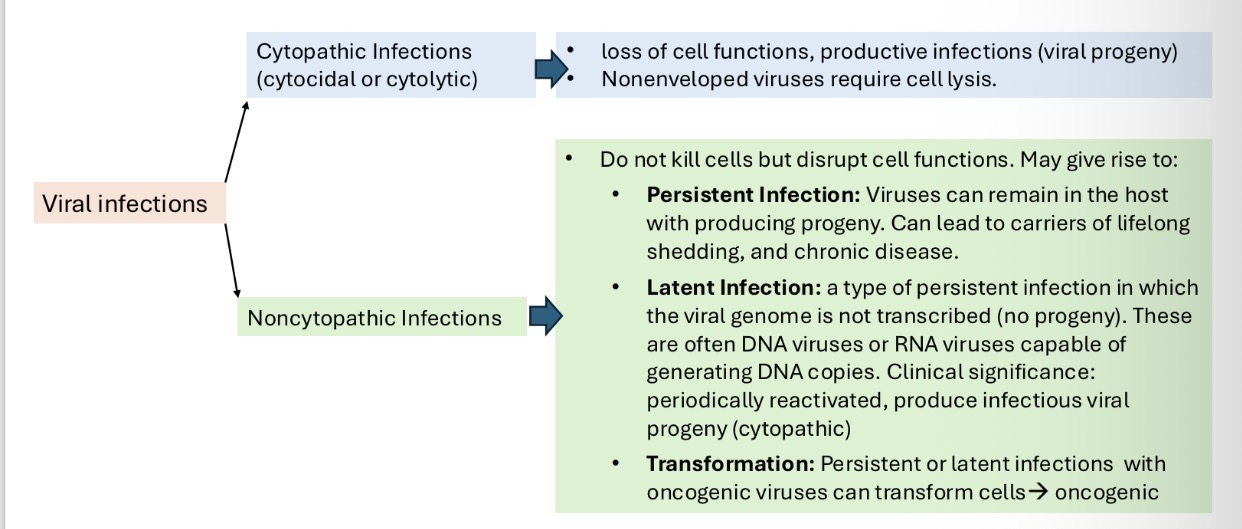
What are cytoplasmic effects in virus-infected cells?
Cytopathic effects (CPE):When cells are infected by a virus, they undergo biochemical and structural changes, known as cytopathic effects. Some CPE are characteristic of a particular virus. can be visible under a microscope → diagnostic value in identifying the infecting virus.
Cytopathic effects:
1. Inhibition of host cell protein production
2. Interference with cell membrane
3. Disruption of cell cytoskeleton
4. Virus-induced metabolic and toxic insult (necrosis)
5. Host defense mechanism (apoptosis)
What are inclusion bodies?
Inclusion Bodies: Visible structures in virus-infected cells, where viral transcription and genome replication occur.
Types of inclusions:
• Intranuclear inclusions: Chromatin margination with staining
• Seen in DNA viruses: herpesviruses and adenoviruses
• Exception (RNA Viruses): canine distemper virus
• Cytoplasmic inclusions: High cytoplasmic replication
• Seen in Poxviruses, Paramyxoviruses
What are the cytoplasmic changes that occur in virus-infected cells?
Inhibition of mRNA transcription, processing, and translation as well as host cell protein production
▪ Rapid and profound shutdown: in picornaviruses, influenza viruses
▪ Gradual shutdown or no shutdown: in nonchtocidal viruses (pestiviruses, retroviruses)
Outcomes:
loss of cellular homeostasis → degeneration and necrosis
• most common early change: cloudy swelling (nucleus becomes condensed and shrunken and cytoplasmic density increases)
• Cell destruction
Interference with cell membrane
a. Increase membrane permeability
b. Proliferate specific membrane structures
c. Multinucleated syncytium formation:
• Enveloped viruses insert their fusion proteins into host-cell membranes, membrane fusion between infected and uninfected cells→syncytia
• Role of syncytia: spread between cells, evading immune detection
Many viral proteins have the potential to bind glycoproteins on the surface of RBCs in a species-specific manner. This can be used as diagnostic tool for virus detection:
• Hemadsorption: RBCs sticking to infected cells → diagnostic marker.
• In influenza viruses, paramyxovirus
• Hemagglutination: RBC clumping due to free virus → diagnostic assay.
Disruption of the cell cytoskeleton
• Responsible for the structural integrity of the cell, for the transport of organelles through the cell, and for certain cell motility activities. Changes in cell shape → precede cell lysis in many infections
Apoptosis (programmed cell death)
• Activation of host-cell caspase enzymes → degradation of the cell’s own DNA and proteins, removal by phagocytic cells. Mechanism of cell suicide:
• The intrinsic pathway: leakage of mitochondrial proteins into the cytosol where these proteins activate cellular caspases.
• The extrinsic pathway: activated by engagement of specific cell-membrane death receptors
What are non-cytopathic changes in virus-infected cells?
These infections do not kill infected cells outright. Instead, cause persistent infection (PI) and produce and release virions.
Maintain normal or minimally disrupted cellular metabolism. Cell continue to grow and divide. In RNA viruses like Pestiviruses (bovine viral diarrhea virus), Retroviruses.
Outcomes:
• In most cases, slowly progressed changes in infected cells ultimately lead to cell death.
• In rapidly dividing tissues (intestinal lining, skin), can replace quickly.
• In terminally differentiated cells (neurons), destroyed cells cannot be replaced (interfere with specialisedfunctions of differentiated cells).
How does viral infection of the respiratory tract occur?
Tropism of Respiratory Viruses is determined by:
• Receptor distribution: The presence of specific receptors on host cells.
• Intracellular factors: Transcriptional enhancers for viral replication.
• Environmental factors: Temperature, barriers, and immune responses.
Respiratory virus tropism:
• Bovine rhinitis viruses (Picornaviridae): Infect the nasal passages because their replication is optimised at lower temperatures → Result in mild rhinitis.
• Influenza viruses: Replicate in both the nasal passages and airways, but confined to the lung (requirement for HA cleavage by tissue-specific proteases)
• Highly pathogenicity → Systemic
• Low pathogenicity→ Confined to respiratory and gastrointestinal tracts
What are the mechanisms of virus-induced respiratory tract tissue damage?
Mechanisms of respiratory tract damage:
• Cessation of ciliary activity
• Loss of mucosal integrity
• Inflammation: block airways, cause hypoxia and respiratory distress
• Secondary or polymicrobial infections due to facilitating factors:
. Stress-induced immunosuppression
. Exposure to ammonia vapors, which impairs ciliary function.
. Crowded, humid conditions that enhance aerosol transmission of enveloped viruses.
What are the modes of viral infection of the GIT:
Modes of viral infection in the GIT:
1. Local GI infection (enteric viruses):
. Viruses such as coronaviruses are ingested and infect only the GIT.
. Often cause rapid-onset diarrhea after a short incubation period.
2. Generalized hematogenous viral infection:
. Viruses like parvoviruses, pestiviruses (bovine viral diarrhea virus) spread to the GIT through the bloodstream during a generalised infection.
. Have a longer incubation period and involve symptoms affecting multiple systems beyond the GIT.
What are the mechanisms of virus-induced tissue damage in the GIT?
Mechanisms of virus-induced diarrhea:
Cellular damage to enterocytes → Malabsorption diarrhea, fluid and electrolyte loss.
Additional effect: Rotavirus cause intestinal hypersecretion
Clinical consequences of severe diarrhea:
• Dehydration
• Hemoconcentration
• Acidosis (metabolic disturbance that impairs critical enzyme activity)
• Hypoglycemia
• Electrolyte disturbances: Low sodium and high potassium due to malabsorption and intestinal hypersecretion
• Outcome: Rapid progression to death in neonates or compromised animals
What are the routes of viral entry to the skin?
Routes of Viral Entry to the Skin:
• Primary infection: Some viruses initially infect the skin at the site of entry (e.g., through cuts or insect bites). →Localized lesions like papilloma
• Secondary infection: Other viruses invade the skin via the bloodstream during systemic spread → Disseminated lesions
What are the types of lesions caused by viruses:
• Macules: Flat, discoloured areas.
• Papules: Raised areas of skin.
• Vesicles: Fluid-filled raised areas.
• Pustules: Raised areas containing leukocytes
What are the mechanisms of virus-induced tissue damage in the skin?
Characteristic cutaneous lesions caused by specific viral families, and in certain locations on body, particularly in ungulates:
• Vesicles (blisters): fluid accumulation within the epidermis, vesicle rupture leaves ulcers
. Vesicles are notable for their association with reportable livestock diseases. E.g. foot-and-mouth disease (FMD)
• Papules: characteristics of poxvirus lesions
. Can be localized (Orf) or widespread (Lumpy skin disease)
. Often become raised and encrusted with inflammatory exudate
What are the routes of viral spread to the central nervous system?
Routes of viral spread to the CNS:
• Via Nerves: Infection of olfactory neurons in the nasal cavity or Peripheral nerve endings
• Via the Bloodstream: Viruses must cross the blood-brain barrier (BBB) or the blood-cerebrospinal fluid (CSF) barrier
What are virus specific CNS pathologies?
• Rabies: noncytocidal, little inflammation but interfering with synaptic signalling, lethal
• Canine distemper: progressive demyelination via glial cell infection
What is the hematopoietic system?
• Myeloid tissues: Bone marrow and cells derived from it (e.g., RBCs, platelets, monocytes, granulocytes).
• Lymphoid tissues: Thymus, lymph nodes, spleen, MALT, and the cloacal bursa in birds.
How do viruses target the hemopoietic system?
Tropism to mononuclear phagocytes→ spread to lymphoid tissues: e.g. bluetongue viruses
What are the consequences of viral infections of the hemopoietic system?
• Acquired Immunodeficiency. Example: FIV in cats
• Generalized Immunosuppression→ enhanced virus replication, classical swine fever virus, bovine viral diarrhea virus, canine distemper virus, feline and canine parvoviruses
How does viral infection of the fetus occur?
• If virus does not cross the placenta,
. Severe maternal infections can lead to fetal death and abortion without the fetus being infected.
• If viruses cross the placenta:
. This happens more frequently in young dams (e.g., First-time mothers) lacking immunity due to:
. No prior vaccination.
. No prior natural infection (prior exposure).
• Teratogenic viruses can cause developmental defects by disrupting organogenesis or damaging progenitor cells. (e.g., BVDV, parvovirus).
What is the outcome of a fetal viral infections?
Outcome of fetal viral infection depends on:
1. Virulence and tropism of the virus
2. The gestational age of the fetus
• Influences stages of organogenesis and degree of immune competence.
. Early gestation: often lead to fetal death, resorption or abortion.
. Sheep: Pregnancy dependent on fetal progesterone, more prone to abortion.
. Swine: Pregnancy maintained by maternal progesterone, less prone to abortion.
• Mid gestation: immune competence is developed by mid-gestation
. Infections before this stage lead to a weak and ineffectual immune response
that leads to persistent infection in bovine viral diarrhea virus in cattle
. Congenital defects or lesions depending on the age
What is the importance of persistent infections?
• May be reactivated, causing recurrent disease
• Lead to immune-mediated disease or neoplasia
• Allow viruses to survive in herd even after vaccination
• Serve as key sources of viral transmission over long distances or after eradication efforts
What are latent infections?
Virus is not detectable except during reactivation. Reactivation stimulated by immunosuppression, stress or hormone
e.g. Bovine Herpes virus 1, Marek’s disease
What are the different clinical categories of viral infections?
• Acute self-limited infection (Rotavirus diarrhoea)
• Persistent:
. Latent infections with episodic reactivation.
. Chronic infections: acute phase followed by faster progression of persistent disease (e.g. canine distemper in CNS).
. Slow infections: subclinical start, gradual progression.
✓ Many persistent viruses show mixed patterns (e.g. retroviruses show both persistence and latency).
What is virus-induced neoplasia?
• Viruses are classified as tumour viruses if part of the viral genome is present in tumours, with expression within the tumour of some viral genes
• DNA viruses can cause neoplasia by inhibiting tumour suppressor genes whereas RNA viruses typically activate protooncogenes
• Causes of genetic changes leading to neoplasia:
• Naturally occurring mutations.
• Exposure to chemical or physical agents.
• Infectious agents, including viruses.
All involve disruption of key cellular pathways regulating growth
How does neoplasia occur?
Neoplasia arises from the clonal expansion of cells with genetic damage in key regulatory genes:
1. Proto-oncogenes: genes that regulate growth and differentiation.
2. Tumor suppressor genes: Inhibit growth by regulating the cell cycle.
3. Apoptosis-regulating genes: Control programmed cell death.
4. DNA repair genes: Ensure genomic integrity.
How do oncogenic RNA viruses induce neoplasia?
Integration into host genome:
• Retroviruses use reverse transcriptase to convert their RNA into DNA and integrate into the host genome. However, in rare cases, this integration disrupts or alters the host normal cellular processes, leading to oncogenesis
Oncogenic retroviruses:
• Acute transforming retroviruses: Directly introduce viral oncogenes into host cells.
• Chronic transforming retroviruses: Indirectly activate host proto-oncogenes by disrupting their normal regulation
How do oncogenic DNA viruses induce neoplasia?
Apart from retroviruses, the most important oncogenic viruses in animals are DNA viruses (Papillomaviruses, Polyomaviruses, Herpesviruses)
Two ways DNA viruses interact with host cells:
1. Productive infection:
• The virus completes its replication cycle within the host cell, leading to cell lysis, not oncogenic.
2. Nonproductive infection:
• The virus does not complete its replication cycle but instead transforms the cell, proliferate uncontrollably.
• In this scenario the viral genome is either integrated into the host DNA or exists independently as a plasmid within the host cell.