ThermoChemistry
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
atomic radius
distance from the centre if nucleus to the outermost electron
ionisation energy
the energy required to remove one oml of the least tightly held electron from one mol of gaseous atoms
electronegaivity
the ability of an atom to attract bonding electrons.
periodic trends across a period
Increase in Nuclear Charge: As you move across a period from left to right in the periodic table, the number of protons in the nucleus increases. This increase in nuclear charge leads to a stronger attraction between the nucleus and the valence electrons.
Constant Number of Shells: the number of electron shells remains constant across a period. and therefore the shielding effect is the same.
Stronger Electrostatic Attraction: The stronger nuclear charge without additional shielding results in a stronger electrostatic attraction between the nucleus and the valence electrons.
Consequences:
Smaller Atomic Radius: The stronger attraction between the nucleus and the valence electrons pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
Higher Ionization Energy: More energy is required to remove an electron, as the attraction between the nucleus and the valence electrons is stronger.
Higher Electronegativity: The ability of an atom to attract electrons in a bond increases due to the stronger nuclear attraction.
periodic trends down a group
Increase in Number of Shells: As you move down a group in the periodic table, the number of electron shells increases.
Distance and Electrostatic Force: The increase in shells results in a greater distance between the nucleus and the valence electrons. This distance weakens the electrostatic attraction between the positively charged nucleus and the negatively charged valence electrons.
Shielding Effect: Additional inner shells create a shielding effect, further reducing the attraction between the nucleus and the valence electrons.
Effect of Increased Protons: Although the number of protons (and hence the nuclear charge) increases down a group, the effect of increased shells and shielding outweighs this. As a result, the overall electrostatic force of attraction decreases.
Consequences:
Larger Atomic Radius: The decrease in electrostatic attraction allows the valence electrons to be further from the nucleus, increasing the atomic radius.
Lower Ionization Energy: Less energy is required to remove an electron, as the attraction between the nucleus and the valence electron is weaker.
Lower Electronegativity: The ability of an atom to attract electrons in a bond decreases as the attraction between the nucleus and the valence electrons weakens.
atomic vs cation (+ve) radii. eg Al and Al 3+
both have the same number of protons
the cation has one less shell than the atom
therefore the atom will have a greater shielding effect (inner shell e-e repulsion) than the cation
this decreases the atoms attraction between the nuclues and valence electrons resulting in the redius of al being greater than al3+
as al3+ has a lesser shielding effect, thus a stronger attraction between the nucleus and valuence electrons and a leer radius.
atomic vs anion eg Cl and Cl-
both atom and anion haved the same number of protons and therefore the same nuclear charge.
both have the same number of shells and therefore the same shielding effects and number of inner shells
however in the valence chell the anion has more electrons
hence there are more e-e repulpision and the e move further apart
therefore the anion has a larger radius than the anion
explaining shape
there are _ areas of electron density around the central atom _
these areas occupy positions to minimise repulsions and therefore maximise separation
this results in the parent shape -
there are_ bonded areas and _ non-bonded areas resulting in the molecular shape _
explaining polarity
the molecule contains polar bonds because __ is more electron negative than _. this results in the electrons being shared unevenly and bond dipoles form. because the shape ( ) is A/Symmetrical the bond dipoles are arranges A/Symmetrical and therefore the bond dipole don’t/do cancel and therefore the molecule is polar/non-polar
what shapes are symmetrical
trigonal bipyramidal, octahedral, linear, square planar
explain polarity in a mixed-polar molecule
contains (-) polar bonds as (-1) is more electronegative than (-2) and (-1) is more electronegative than (-3). Even though the shape (-) is symmetrical, the bond dipoles are different due to the different electronegativity of (-2) and (-3) and therefore the bond dipoles do not cancel and the molecule is polar.
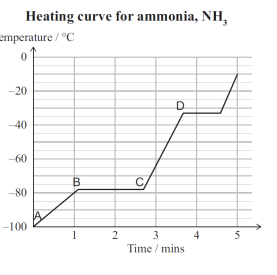
explain this heating curve (plus adding E at the end of the latent heat of vaporization and F at the top)
between a and b as the solution is heated the ammonia molecules in a solid state gain kinetic energy, and therefore the molecules will vibrate/move to a greater extend and the intermolecular forces between them will become weaker. because Ek has increased the temperature has increase.
between b and c the added heat energy is used to break some of the intermolecular attractions between the ammonia molecules when changing state from a solid to a liquid/melting. and therefore the average kinetic energy if the particles remains the same and therefor the temperature also remains the same
between c and d, ammonia molecule in liquid state gain kinetic energy, so the temperature increases. due to the increase in kinetic energy the ammonia molecules move faster and further apart
between D and E, the added heat energy is used to break intermolecular attractions between the molecules and therefore separe the ammonia molecules from one another in the change of state from a liquid to a gas. since the kinetic energy of the ammonia molecules remains constant, the temperature also remains constant.
between E and F, Ammonia molecules in gas state gain kinetic energy so the temperature increases, due to the increase in kinetic energy the ammion molecules move faster/very fast
what is ΔH
the difference in the total energy of products and reactants in KJmol-1, under standard conditions
what is enthalpy of formation
the enthalpy change when one mol of a substance is formed from its elements in their standard states
what is enthalpy of combustion
the energy released when one mol of a substance is completely combusted in O2(g)
what is enthalpy of vapourisation
the energy required to turn one mol of liquid to one mol of gas at its boiling point in standard conditions
what is enthalpy of fusion
the energy required to turn one mol of solid to a liquid at its melting point under standard conditions.
what is hess’s law
hess’s law states that the enthalpy change for a reaction is independent of the number of steps taken and depends only on the amount and type of reactants and products.
what is entropy
the tendency of the universe to move towards disorder and decay
nature is lazy - objects and systems tend to lower energy states
nature is messy -objects and system get more disordered
change in entropy of the surrounds (endo and exo reaction)
Exothermic Reactions:
Energy Release: In an exothermic reaction, energy is released from the system to the surroundings, usually in the form of heat.
Impact on Surroundings: This release of energy increases the kinetic energy of the surrounding particles, causing them to move faster and become more disordered.
Entropy Change: As a result, the entropy of the surroundings increases because there is a greater dispersal of energy and matter. Therefore, the change in entropy of the surroundings is positive.
Endothermic Reactions:
Energy Absorption: In an endothermic reaction, energy is absorbed from the surroundings into the system.
Impact on Surroundings: This absorption of energy decreases the kinetic energy of the surrounding particles, causing them to move slower and become less disordered.
Entropy Change: As a result, the entropy of the surroundings decreases because there is a lesser dispersal of energy and matter. Therefore, the change in entropy of the surroundings is negative.
change in entropy of the system
entropy of system will increase when
state change solid→liquid→gas
there are more moles of particles formed in the products then there were in the reactants
a solid is dissolved in water to make an aqueous solution
the volume of the system increases
in all the examples above there is a greater dispersal of matter and energy this results in more disorder and a positive enthalpy change.
justifying why a reaction is spontaneous when both enthalpy of the system and surroundings are positive
since the entropy of both the system and the surrounding increases, this means the total entropy change will be positive and therefore the reaction will be spontaneous.
justifying why a reaction is spontaneous when enthalpy of the system and surroundings are opposite (one positive and one negative)
the increase in entropy of (the positive one) must outweigh the decrease in entropy of the (negative one) making the overall entropy change positive and therefore the reaction will be spontaneous.
what type of WIF do non-polar molecules have
temporary dipole - temporary dipoles
what type of WIF do polar molecules have
temporary dipole - temporary dipoles
permanent dipole- permanent dipole
(H bonding if it has F/O/N to a H)
how can the shape effect the BP
linear molecules pack closer together therefore the Td-Td are stronger and their BP will be higher
bulky/branched/spherical molecules cannot pack as closely and therefore their td-td are weaker as are their boiling points.
explain the why the boiling point of propan-1-amine is higher than chloroethane (H-bonding)
propan-1-amine and chloroethane both have electron clouds of similar size and therefore the temporary dipole attractions between the molecules are of similar strength.
both chloroethane and propan-1-amine have permanent dipole attraction between their molecules (due to the C-Cl dipole and the N-H dipole, respectively).
However, propan-1-amine also has hydrogen bonding between its molecules, due to the large difference in electronegativity between N and H. as a result more heat energy is required to overcome the intermolecular forces between propan-1-amine so it has a higher boiling point than chloroethane
comparing decane and chloroethane
chloroethane has permanent dipole attractions between its molecules (due out the c-cl dipole), as well as temporary dipole attractions.
however since decane has a much larger electron cloud dur to its larger molar mass, the sum of its temporary dipole interactions is greater, so more heat energy is required to separate decane molecules compared to chloroethane molecules. as a result, decane has a higher boiling point than chloroethane.