Ch20: Carbs' Structure & Function
1/122
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
123 Terms
Carbohydrates are organic compounds made up______-, and they serve as a key source of energy for the body.
of carbon, hydrogen, and oxygen
4 main categories of carbs
reflecting increasing complexity
monosaccharides
disaccharides
oligosaccharides
polysaccharides
sugar unit # and examples: monosaccharides
1
Glucose, Fructose, Galactose
sugar unit # and examples: disaccharides
2
sucrose, lactose
sugar unit # and examples: oligosaccharides
3-10
raffinose, stachyose
sugar unit # and examples: polysaccharides
>10
starch, cellulose, glycogen
simplest form of carbohydrates, consisting of a single sugar unit.
monosaccharides
a primary energy source for cells
Glucose
part of lactose in dairy products
Galactose
sugar found in fruits
Fructose
Monosaccharides are absorbed directly in the ______ into the bloodstream and are quickly used for energy
small intestine
ID this structure:
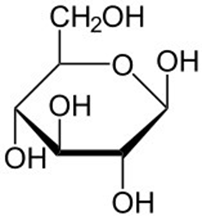
glucose
ID this structure:
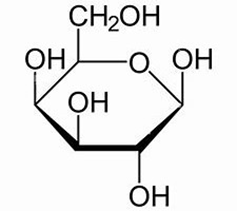
galactose
ID this structure:
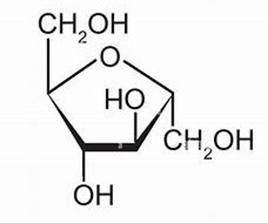
fructose
glucose and galactose are _____ of each other
isomers
Monosaccharides, often referred to as simple sugars, are polyalcohols that contain either an ________ or a ________. Structurally, they can exist in both _______ forms.
aldehyde (called aldose)
ketone group (called ketose)
straight-chain and ring
Disaccharides are formed by two monosaccharide molecules linked together by a _______ bond.
glycosidic
Give this disaccharide’s common name/formation & its sugar subunit: sucrose
table sugar
a-D-glucose – b-D-fructose
Give this disaccharide’s common name/formation & its sugar subunit: lactose
milk sugar
b-D-galactose – b-D-glucose
Give this disaccharide’s common name/formation & its sugar subunit: maltose
formed during the breakdown of starch
a-D-glucose – a-D-glucose
Disaccharides must be broken down into monosaccharides by ________ before they can be absorbed and used by the body
enzymes
________ are often found in certain vegetables, legumes, and grains, which are not fully digested by humans and can cause gas when consumed, as they are fermented by bacteria in the large intestine.
Oligosaccharides
Oligosaccharides covalently modify (via attachment) proteins and lipids to generate _________ involved in cell recognition and signaling
glycoproteins and glycolipids
In glycoproteins, they are bonded to specific ______, while in glycolipids, they are linked to _______molecules
amino acid residues
lipid
________ are common membrane components with carbohydrate chains exposed on the extracellular surface for recognition and signaling
Glycoproteins and glycolipids
_______are large, complex carbohydrates made up of many monosaccharide units linked together
Polysaccharides
________, a storage form of glucose in plants
Starch
___________, the storage form of glucose in animals that are found mainly in the liver and muscles
Glycogen
__________, a major structural component of plant cell walls that is not digestible by humans but acts as dietary fibers
Cellulose
___________ are composed of glucose units but differ in their anomeric forms, their structures, function, and the types of bonds that link the glucose units together
Starch, glycogen, and cellulose
Energy production – _______are the major source of energy for our body
Carbohydrates
Energy storage - Excess ________is stored as glycogen (the majority of which is stored in the muscle and liver) providing energy in between meals or during intense activity
glucose
Building blocks of macromolecules: ________ are essential building blocks of important macromolecules, such as RNA, DNA, NAD, FAD and ATP
ribose and deoxyribose
Cell Recognition and Signaling: Cell surface __________ are crucial for recognition and signaling, participating in cell adhesion, immune responses, and intercellular communication
glycoproteins and glycolipids
Systematic Names of Monosaccharides: Monosaccharides are named according to the presence of either an aldehyde (prefix _________) or ketone (prefix _______) functional group, the carbon chain length, and usually conclude with the suffix __________
Aldo-
Keto-
-ose
Monosaccharides typically have a carbon count ranging from ________—
three to six
Trioses with 3 carbons are the smallest _______
monosaccharides
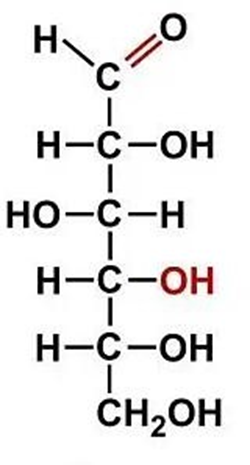
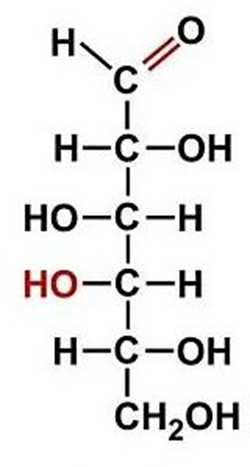
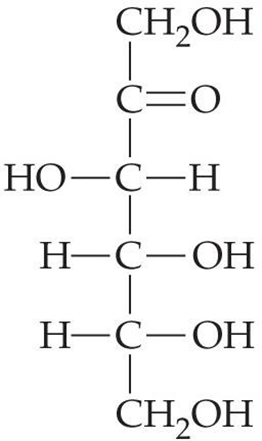
Formula C3H6O3 that can correspond to two sugars: ________________. These two sugars are constitutional isomers.
Aldotriose (AKA Glyceraldehyde) and Ketotriose (Dihydroxyacetone)
ID this structure’s trivial name:
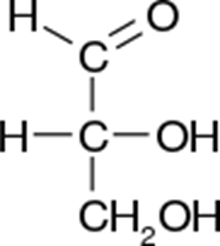
glyceraldehyde (intermediate in glycolysis)
ID this structure’s trivial name:
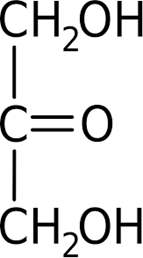
dihydroxyacetone (intermediate in glycolysis)
Sugars are predominantly recognized by their _______ names, each unique to a specific sugar
common or trivial
The limitation of trivial names is their lack of information regarding the sugar's __________
structure, carbon count, or functional groups
Carbohydrates has a general formula _______, with exceptions
(CH2O)n
________are compounds that share identical molecular and structural formulas but exhibit distinct spatial arrangements of atoms, resulting in different shapes
Stereoisomers
________are stereoisomers that are not mirror images of each other and differ at one or more (but not all) chiral centers
Diastereomers
achiral means?
not chiral (duhhhh lol)
_______carbon is a carbon that bonds to four different groups
Chiral
A structure with n stereocenters will have a maximum of _________ different stereoisomers
2n
Glyceraldehyde has two stereoisomers; more specific they are _______; non-superimposable mirror images
enantiomers
In conventional naming, a sugar is designated as an ____ if the –OH group on the last chiral carbon is on the left, and as a __________ if it is on the right. Only the _____ on chiral carbons are considered when identifying mirror images
L-isomer
D-isomer
–OH groups
The prevalent form for naturally occurring sugars is the _______
D-form
In a molecule with multiple chiral carbons, the chiral carbon ______ is chosen for determining the L- or D-forms
farthest from the carbonyl carbon
_______are stereoisomeric pair that serves as mirror images of one another. They share identical properties, trivial names, but vary based on the L- or D-form. Examples: _______
Enantiomers
D-Allose and L-Allose; D-Glucose and L-Glucose
_______specifically refer to stereoisomers differing solely in the absolute configuration at a singular chiral center known as the ______ carbon. Examples: ________
Epimers
epimeric
D-Allose and D-Glucose are C3 epimers
An alcohol group reacts with a carbonyl group to form a _____; compounds with –OH and –OR bond to what was once the carbonyl carbon of an aldehyde/ketone
hemiacetal / hemiketal
How does an alcohol group react with a carbonyl group to form a hemiacetal/hemiketal? (i.e., what is it about these two groups that would encourage this arrangement?)
Alcohols (ROH) are weak acids, and carbonyl groups are polar (O partial(-) & C partial(+)).
Case of opposites attract: Alcohol H+ bonds to Carbonyl O-, and Alcohol RO- bonds to Carbonyl C+.
The open-chain, monosaccharide form is mostly converted to _______ form (more than 99% in a mixture of aqueous solution).
cyclic hemiacetal (rings)
The carbonyl carbon is called the ____ carbon in the ring form.
anomeric
Glucose contains both alcohol and aldehyde functional groups that readily undergo an ________- reaction.
intramolecular addition
Depending on how the ring was closed, D-Glucose can form two molecules: ____________. The two forms are called _______ since they are isomers at the _______ center/carbon.
α-D-Glucose and β-D-Glucose
"anomers"
anomeric
In the α-form of cyclic glucose, the -OH group at the anomeric center is on the _________ the -CH2OH group
opposite side to
In the β-form of cyclic glucose, the -OH group at the anomeric center is on the _________ the -CH2OH group
same side (besides) as
Mutarotation is the spontaneous change in the specific ________of a sugar solution due to interconversion between its______ forms through the ______ form.
optical rotation (OR)
α- and β-anomeric
open-chain
Example: a-D-Glucose ←→ Open-chain form ←→ b-D-Glucose
Simple sugars, such as glucose and fructose, predominantly adopt ring/cyclic structures when in a solution. Only the cyclic form, as opposed to the open-chain form, participates in ________.
polymerization
D-Glucose (C6H12O6) AKA blood sugar, aldohexose, is the most important sugar in human metabolism b/c energy ________.
extraction starts with glycolysis
D-Glucose (C6H12O6) AKA blood sugar, aldohexose, is produced ________in plant, and by _______in humans, and it serves as building block of biopolymers: ______(plants) and ________(animals).
photosynthesis
gluconeogenesis
Starch
glycogen
D-Glucose (C6H12O6) AKA blood sugar, aldohexose, levels in the blood are primarily regulated by two hormones: _______. An imbalance of these, leads to elevated blood glucose levels, associated with ________.
Insulin and glucagon
diabetes
galactose is commonly found in milk as a component of the disaccharide _______, and in glycolipids
lactose
galactose is an epimer of glucose and can be converted into glucose or enter _______—to yield energy when needed.
glycolysis
_______ is a rare genetic disorder due to the deficiency of any enzymes involved in breaking down galactose, which may cause liver failure, mental retardation and cataracts. This is treated by a galactose-free diet
Galactosemia
________ is present in fruits and honey, and can be produced by the hydrolysis of corn starch to make high-fructose corn syrup.
Fructose, also known as fruit sugar,
_______ often serves as a sweetener in beverages and processed foods
Fructose
Phosphorylated fructose is an intermediate in the _______ pathway
glycolytic
_________is primarily metabolized in the liver, where it bypasses key regulatory steps in glycolysis (unlike glucose). This can lead to an overproduction of triglycerides, contributing to _______ (NAFLD).
Fructose
non-alcoholic fatty liver disease
ID this structure:
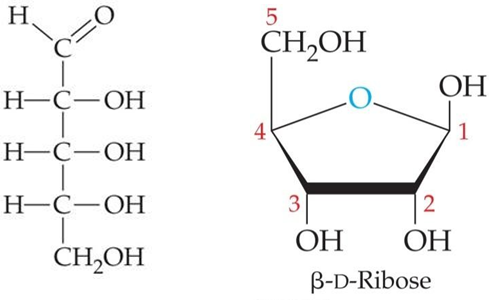
ribose, C5H10O5
ID this structure:
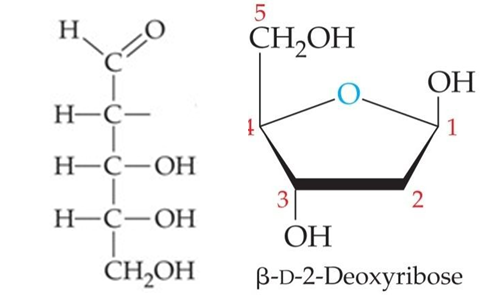
deoxyribose, C5H10O4
________is part of larger biomolecules, such as DNA, RNA, NAD+, FAD, coenzyme A, and ATP.
Ribose
______, w/ the -OH at carbon 2 of ribose replaced by -H (lacking oxygen).
Deoxyribose
Aldoses are _______sugars because they contain an aldehyde functional group that can be oxidized to carboxylic acid.
reducing
In aqueous solution, although aldoses exist primarily in a cyclic hemiacetal form, they are in equilibrium with __________ group. This aldehyde can act as reducing agents by being oxidized.
a small amount of the open-chain form that contains a free aldehyde
In alkaline solution, ketoses undergo rearrangement to become aldoses via the intermediate _________
enediol
An _______is an alkene with an -OH group attached to each of the two carbon atoms forming the double bond.
enediol
Ketoses by converting to aldoses can also act as _________.
reducing sugars
All _______ including both aldoses and ketoses are reducing sugars.
monosaccharides
In a monosaccharide, the…
hemiacetal group + alcohol → acetal + H2O
What is the acetal group called, and what is its significance?
It is called glycoside, and they are no longer reducing sugars b/c the anomeric C is locked in the acetal linkage.
When the…
hemiacetal group of one sugar + hydroxyl group from another sugar → disaccharide + H2O
…what is the linkage called?
glycosidic linkage
_______is formed from rxn b/t a hemiacetal group on one glucose and the hydroxyl group at C4 on another glucose. The linkage is called _____, w/ H2O released in the process.
maltose
α-1,4 glycosidic bond or α-1,4link
Maltose retains a free ______ group at Glucose 2, hence it is a reducing sugar
hemiacetal
Maltose is produced during the enzymatic digestion of ______ (Stage 1 of Food catabolism).
starch and glycogen
ID this structure:
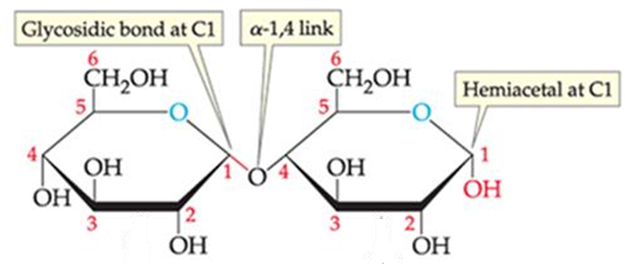
maltose
Glycosidic linkages are named by reading from left to right:
Position (a or b) of the anomeric –OH (left glucose)
followed by numbers that correspond to the locations of the carbons involved in the glycosidic bond (C1 to C4)
Lactose AKA milk sugar, constituting 2-8% of human milk by weight and comprises a ______ bond.
β-D-galactose linked to a β-D-glucose through a β-1,4
Lactose is classified as a reducing sugar due to the presence of a _______
hemiacetal in glucose
Lactose intolerance results from the inability to hydrolyze lactose, caused by an _______. In the colon, normal bacteria interact with undigested lactose, leading to symptoms such as diarrhea, gas, and bloating
enzyme (lactase) deficiency
Patients with _____ should avoid ingesting lactose.
Galactosemia
Sucrose, also known as table sugar, is composed of _______ link.
α-D-glucose and β-D-fructose linked by an α-1,2
The hemiacetal of α-D-glucose reacts with the hemiketal of β-D- fructose.
Sucrose is a ______ because both of its anomeric carbons are involved in the glycosidic bond, leaving no free hemiacetal or hemiketal group.
non-reducing sugar
Sucrose is hydrolyzed in the small intestine by _____ yielding a 50:50 mixtures of glucose and fructose
sucrase
The hydrolysis of disaccharides involves the breaking of the ______ that connects the two monosaccharide units in the disaccharide molecule, resulting in the formation of individual monosaccharides
glycosidic bond
This hydrolysis of disaccharides reaction takes place during digestion of carbohydrates catalyzed by a class of enzyme known as _____. Sugar specific enzymes are named after the sugar.
hydrolases