ionic, covalent, periodic trends in bonding and structure
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
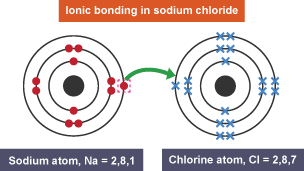
ionic bonding
strong electrostatic attraction between a positive and a negative ion (cation and anion)
it holds cations and anions in ionic compound
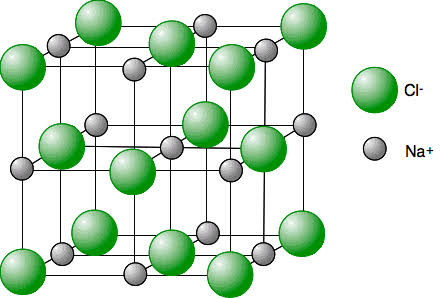
giant ionic lattice
structure containing billions of billions of ions
properties of ionic compounds
most ionic compound have high melting and boiling points (solid at RT)
high temps needed to provide large quantity of energy to overcome the electrostatic attraction between the ions
many ionic compounds dissolve in polar solvents such as water
solid state → not conduct electricity
melted or dissolved in water → does conduct electricity
simple covalent bond
is the strong electrostatic attraction between a shared pair of electrons and the nucleid of the bonded atoms (non-metals)
simple covalent bond properties
Low Melting and Boiling Points:
Covalent bonds form discrete molecules. The forces between these molecules (intermolecular forces) are weak, requiring little energy to break.
Poor Electrical Conductivity:
In most simple covalent substances, there are no free-moving, charged particles (like electrons or ions) to carry an electric current.
insoluble (Often):
Simple molecules often do not dissolve well in water, as they are typically non-polar and cannot interact effectively with polar water molecules.
lone pair
is where paired electrons that arent shared

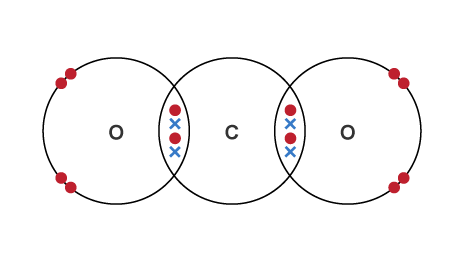
multiple covalent bonds
exist when 2 atoms share more than one pair of electron
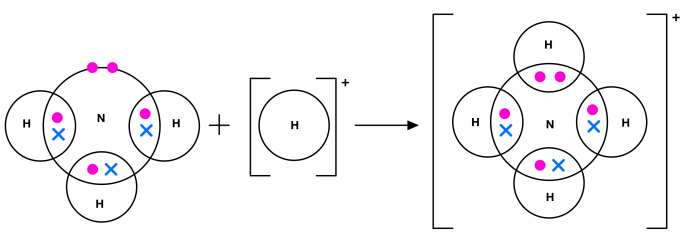
dative covalent/coordinate bond
is a covalent bond in which the shared pair of electrons has been supplied by one of the bonding atoms only
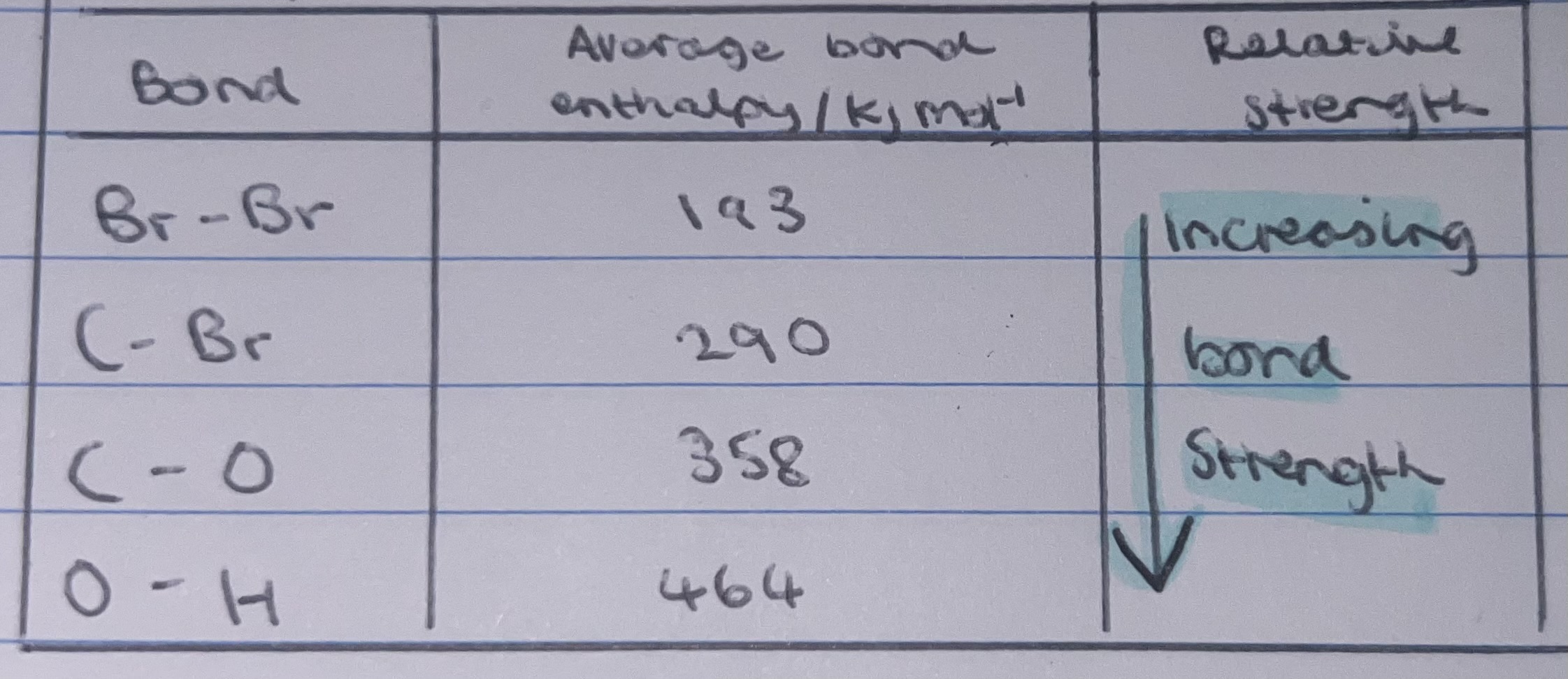
average bond enthalpy
serves a measurement of covalent bond strengths. the larger the value of the average bond enthalpy, the stronger the covalent bond
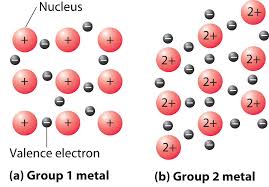
metallic bonding
electrostatic attraction between positive ions and delocalised electrons
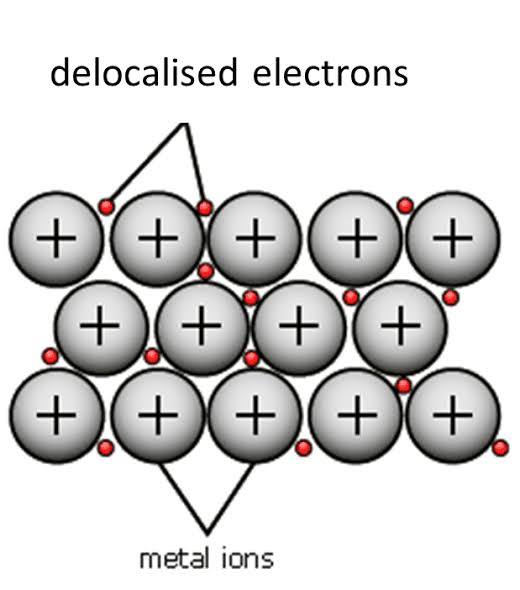
delocalised electrons
that they arent confined to a single atom and is mobile throughout the whole structure
giant metallic lattice
billions of metal atoms are held together by metallic bonding
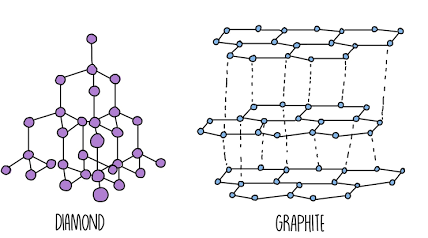
giant covalent structure and its properties
many billions of atoms are held together by a nerwork of strong covalent bonds to form a GCL
eg diamond (carbon)
strong covalent bonds between atoms
very high M/B point (strong C.B)
mostly non-conductors (except graphite)
Insoluble
very hard (dia) or slippery (graphene)
properties of metals
malleable
high m/b points due to strong metallic bonds
great conductors of heat and electricity due to delocalised electrons (that are mobile)
most metals are solid at room temps
allotrope and examples
different structural form of the same chemical element in the same physical state - carbon; diamond, graphite, graphene
Why do melting points increase from Na → Mg → Al in Period 3?
Metallic bonding gets stronger: ion charge increases (+1 → +2 → +3), more delocalised electrons, stronger attraction between ions and electrons.
Why does Si have the highest melting point in Period 3?
It has a giant covalent structure with strong covalent bonds throughout, which need lots of energy to break
Which order do P₄, S₈ and Cl₂ melt?
S₈ > P₄ > Cl₂ (because S₈ is the biggest molecule, Cl₂ the smallest).
Why does Argon have the lowest melting point in Period 3?
Ar exists as single atoms, so only very weak forces between atoms