Topic 2 - Atomic Structure: Electronic Configuration
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
Hydrogen-like Atoms
Definition: Atoms/ions with any nuclear charge but only one electron (e.g., H, He⁺).
Schrödinger equation: Can be solved exactly for these systems.
Coulombic attraction between the nucleus and surrounding electrons increases with the number of protons
Example: H (+1) vs He⁺ (+2).
Effect: Higher nuclear charge → stronger electrostatic force → orbital contraction (electron closer to nucleus) → orbital energy is lower.
Multi-Electron Atoms
Definition: Atoms/ions with multiple electrons
Schrödinger equation: More protons (electrostatic attraction) and electron–electron repulsion make the Schrödinger equation unsolvable exactly.
Use approximation: Assume structure is similar to one-electron atoms, for which there are exact solutions.
Key considerations in approximation:
Fill orbitals from lowest energy upward - achieve the lowest energy electronic structure.
Orbitals with same type & same principal quantum number n are degenerate (equal energy). Example: 2pₓ, 2pᵧ, 2p𝓏.
Rules for Assigning Electrons - Aufbau Principle
Fill orbitals from lowest (most stable orbital) to highest energy (least stable orbital).
In one-electron atoms, orbital energy depends on the principal quantum number n.
In multi-electron atoms, orbital energy depends on the principal quantum number n and the angular momentum quantum number l.
Energy order not strictly by n (e.g., 4s fills before 3d).
Filling order remembered via diagonal rule.
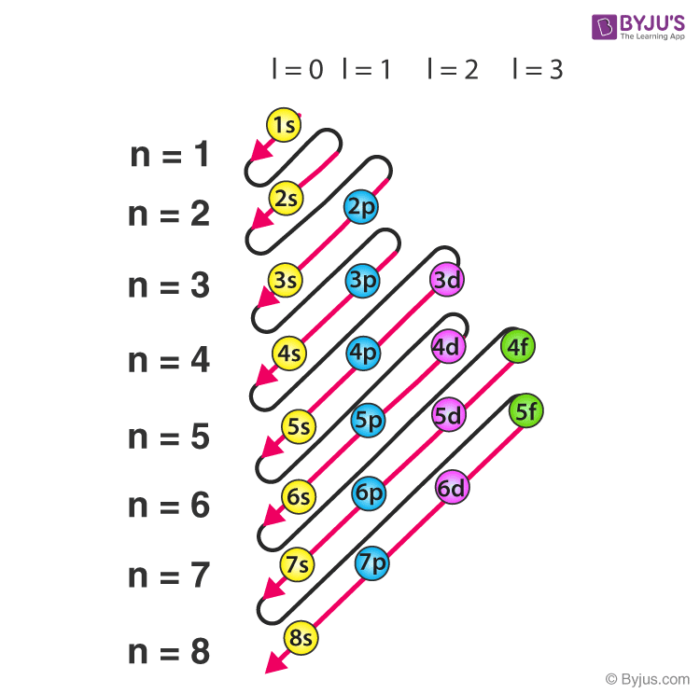
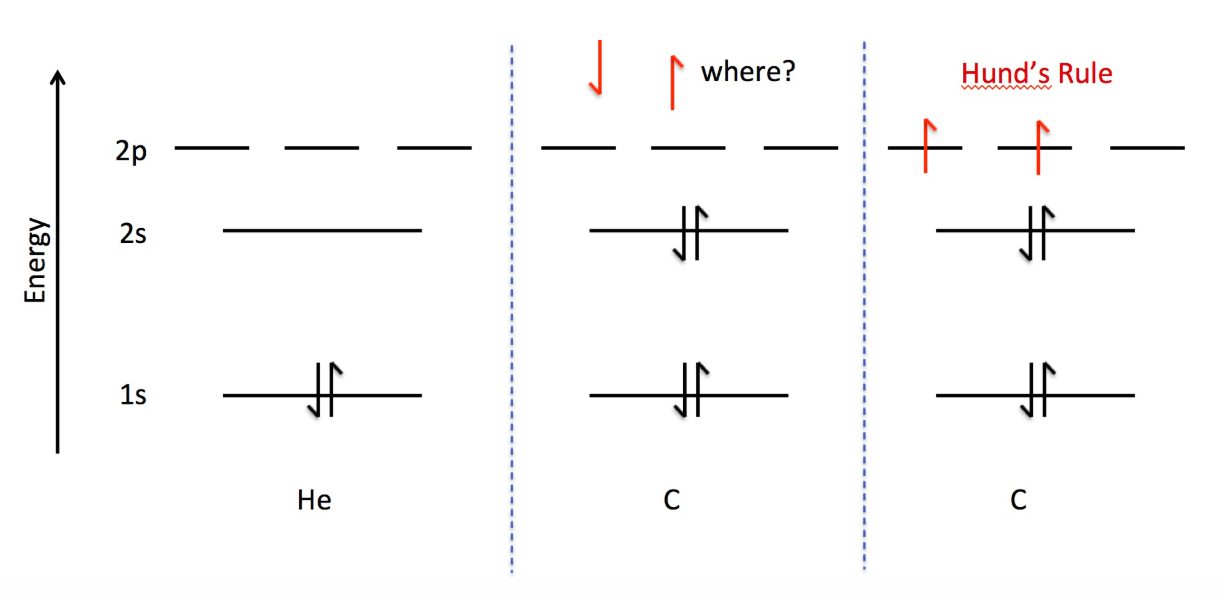
Rules for Assigning Electrons - Hund's Rule
For degenerate orbitals: Orbitals will fill to maximize the electron spin by filling each orbital with one electron with the same spin first, and then pairing each of those electrons up after.
Rules for Assigning Electrons - Pauli Exclusion Principle
Maximum of 2 electrons per orbital, with opposite spins.
Electron Configuration
A representation to show a particular arrangement of electrons within an atom, by orbitals
Ground State Configuration
Lowest possible (and most stable) energy arrangement.
Excited State Configuration
Higher energy than ground state (less stable) configuration.
Full ‘1s’ Notation
Each orbital written as: Xab
- X = principal quantum number
- a = angular momentum letter (s, p, d, f)
- b = total number of electrons in all those (degenerate) orbitals for that specific orbital type of an energy level (Xa)
Abbreviated ‘1s’ Notation
Use nearest noble gas for core electrons, list only valence electrons.
Example: Ne - [He]2s22p6
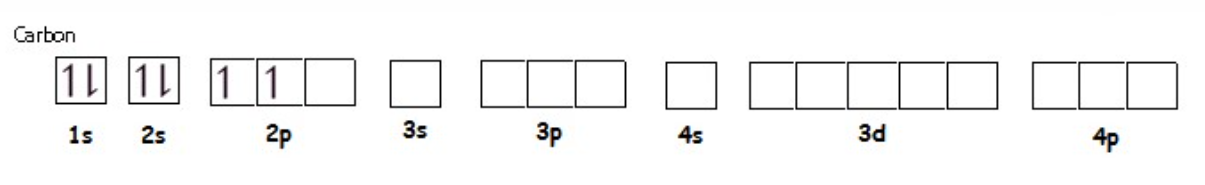
Arrows in Boxes Notation
Orbital filling order (Aufbau principle).
Electron spins (↑↓).
Hund’s Rule (unpaired electrons with same spin first).
Electron Configuration for Ions
Anions: Add electrons to neutral atom’s configuration.
Cations: Remove electrons from highest energy level first.
Examples:
Anion: O²⁻ from O (1s²2s²2p4) → add 2 more electrons → O²⁻ (1s²2s²2p6)
Cation: Mg²⁺ from Mg (1s²2s²2p⁶3s²) → remove 2 electrons → Mg²⁺ (1s²2s²2p⁶).
Isoelectronic Species
Species that share the same electron configuration but are not the same species - same amount of electrons but different nuclear charges.
Example:
Ne: 1s22s22p6
O2-: 1s22s22p6
Mg2+: 1s22s22p6
Magnetic Properties
Paramagnetic: Atom/ion with at least one unpaired electron → attracted to magnetic fields (e.g: carbon).
Diamagnetic: Atom/ion with all electrons paired → repel magnetic fields (e.g: helium).