GC550: Mitochondria Function and Structure: Oxidative Phosphorylation, ETC, Proton Motive Force generation and uncoupling
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
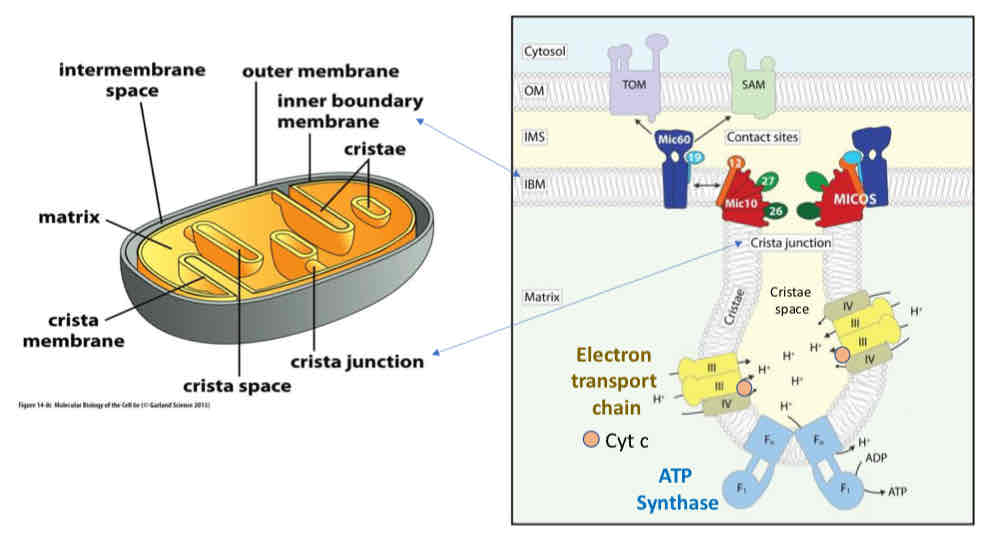
Mitochondrial Structure
Smooth outer membrane and highly convoluted inner membrane, generating multiple distinct mitochondrial compartments: intermembrane space and mitochondrial matrix
Cristae: intermembrane space subcompartment formed by inner membrane folds, connected to the boundary intermembrane space by cristae junctions
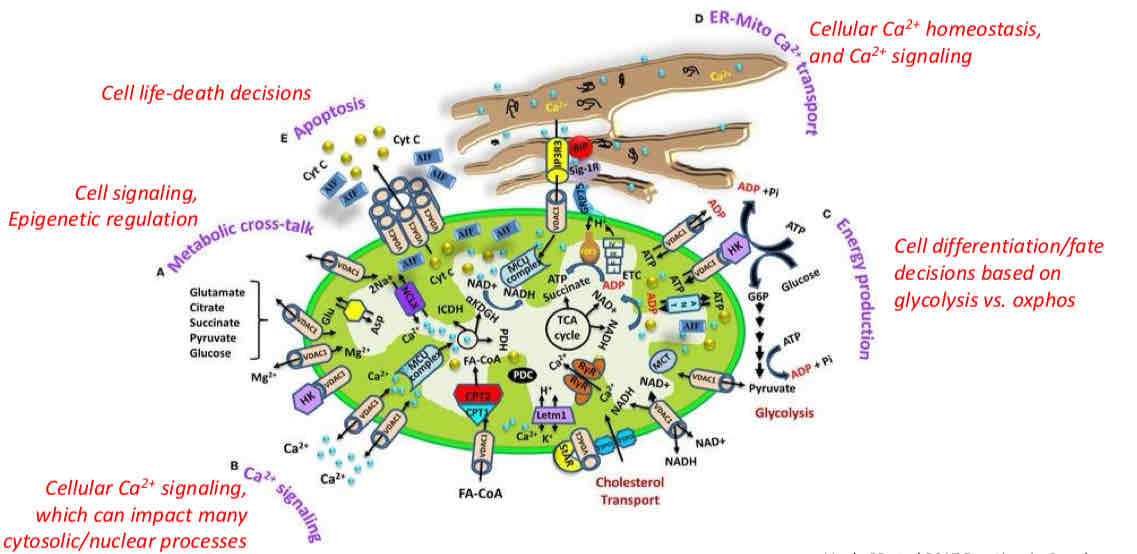
Overview of Mitochndrial Functions
mediate apoptosis (cell life-death decisions)
Cell signaling, epigenetic regulation
Cellular Ca2+ signaling, which can impact many cytosolic/nuclear processes
Make heme and generate urea
Cellular Ca2+ homeostasis and Ca2+ signaling
Metabolite/Ion transport across OMM vs IMM
OMM
contains large diameter pore-like channel VDAC → broad specificity for molecules of MW <5000, open and closed state may be subject to regulation
relatively porous
IMM
not porous, tight
contains transporters and channels for metabolite/ions (Inorganic phosphate, ATP, ADP, Pyruvate, di and tricarbxylic acids, amino acids, long chain acyl carnitine esters, Na+, K+, Ca2+)
Free diffusion of O2 and CO2
highly regulated
We want this to establish the electrochemical gradient necessary to generate ATP
A large extent of H+ transport across IMM is via protein complexes involved in ox phos
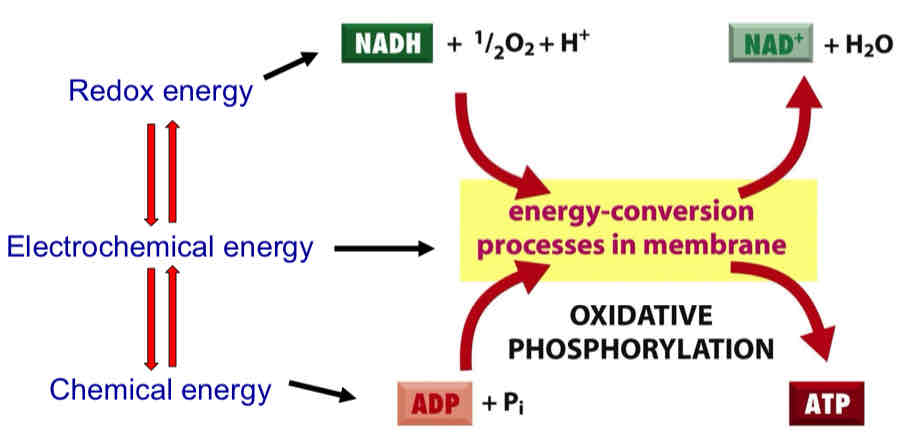
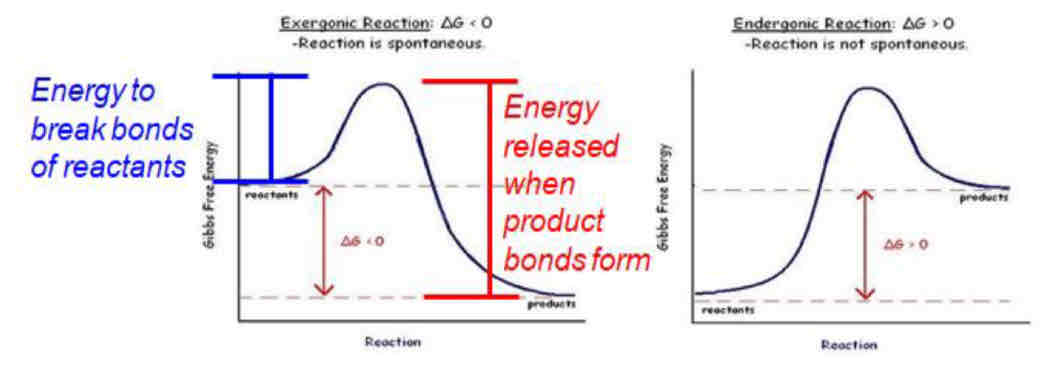
Oxidative Phosphorylation Overview
coupling of substrate dilation and phosphorylation of ADP through a proton electrochemical gradient
ATP Hydrolysis vs ADP phosphorylation
ATP hydrolysis is highly exergonic (releases energy) → energy captured from this reaction can be used to drive endergonic reactions (energy stored in ATP used to drive reactions)
ADP phosphorylation/ATP synthesis is highly endergonic (unfavorable, input of energy)
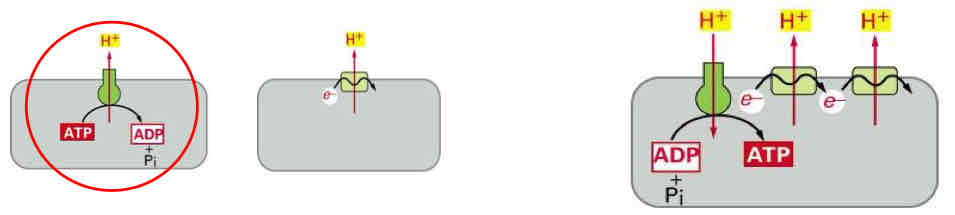
Proton Pumps
key to electrochemical energy conservation
Oxidative phosphorylation relies on two types of proton pump with the same orientation located in the same membrane
ATPase proton pump → energy of ATP hydrolysis can be used to drive electron transport upstream to reduce NAD+ and NADH (reverse of ox phos)
Electron shuttle → driven by the free energy change of redox reactions
Coupling of these pumps (redox-driven proton pump with an ATP-driven proton pump in the same membrane) permits reversal of the ATPase reaction driven by the free energy change of the redox reactions
Free Energy of Membrane Transport Reactions
Uncharged molecular transport → driving force (free energy change) s determined by concentration gradient
Charged molecular transport → driving force is determines by concentration gradient and by the electrochemical potential difference across the membrane
Mitochondrial Proton Motive Force (∆p) → generated by [H+] → concentration gradient (pH) and electrical gradient (charge separation)
Mainly composed of voltage change/charge separation
![<ul><li><p><strong>Uncharged molecular transport</strong> → driving force (free energy change) s determined by concentration gradient </p></li><li><p><strong>Charged molecular transport → </strong>driving force is determines by concentration gradient and by the electrochemical potential difference across the membrane </p></li><li><p><strong>Mitochondrial Proton Motive Force (∆p)</strong> → generated by [H+] → concentration gradient (pH) and electrical gradient (charge separation)</p></li><li><p>Mainly composed of voltage change/charge separation</p></li></ul>](https://knowt-user-attachments.s3.amazonaws.com/77aca0dc-56f9-46f5-8320-eedc80ff6f15.jpg)
Generation of ∆p
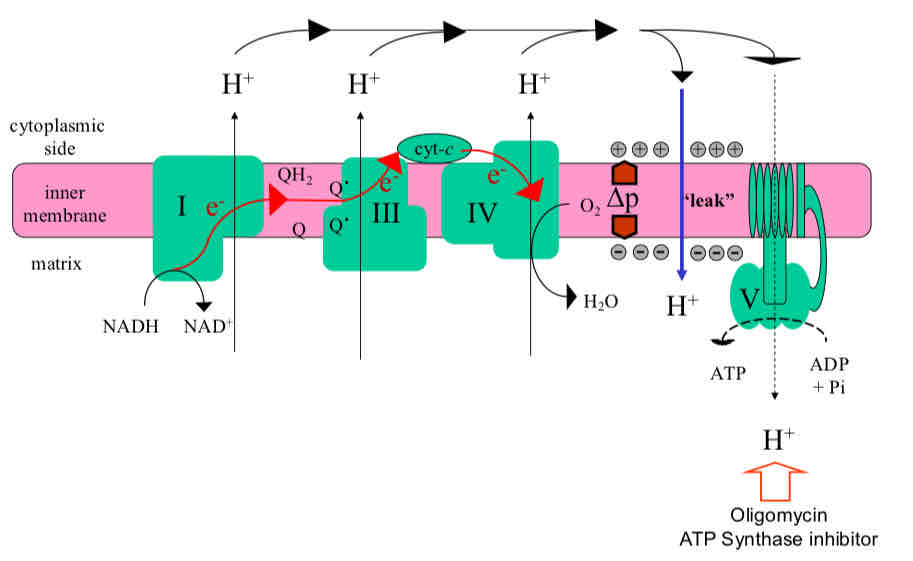
ETC is a chain of redox of reactions that generate free energy to pump protons
Three complexes shuttle electrons → NADH/FADH2 reducing equivalents get oxidized which drives proton pumping in complex I (FADH2 oxidized by complex II but complex II does not pump protons)
Electrons are shuttled by ubiquinone (Q) to complex III, which also drives H+ pumping
Electrons are shuttled to cytochrome c and onto complex IV which also pumps protons
O2 is final e- accepter and water is produced → Liberates free energy that drives proton pumping through complex IV
Electrons enter at Complex I OR II (via NADH or FADH2)
Coupled to CAC through succinate dehydrogenase to Q
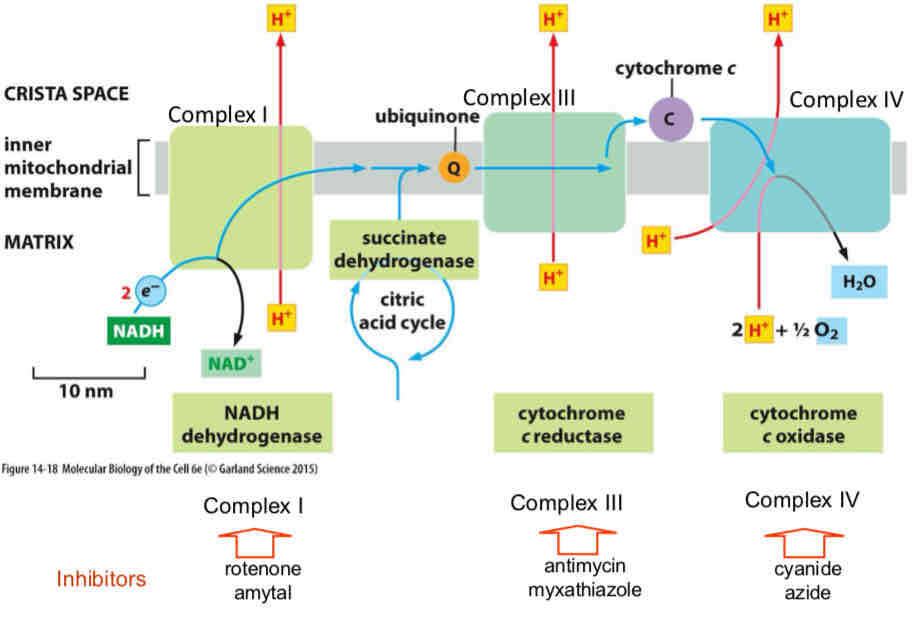
Electron Carriers in the Mitochondrial Respiratory Chain
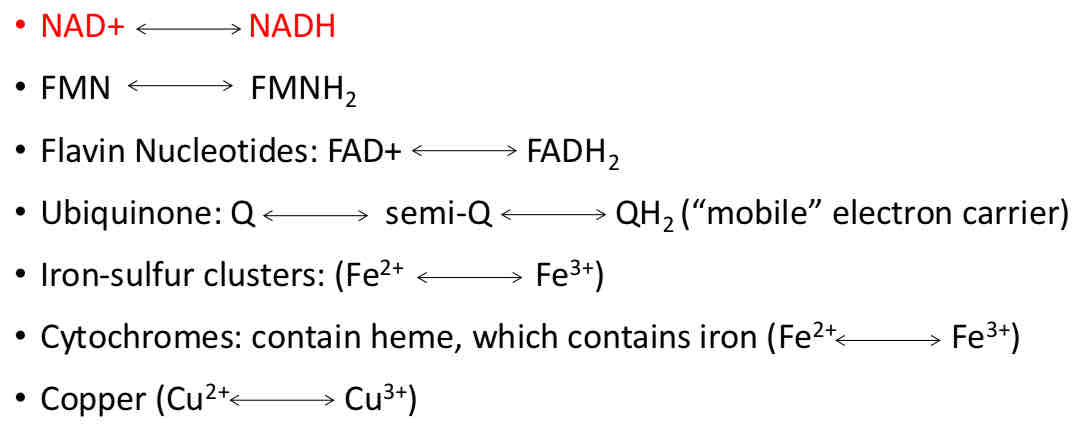
Mitochondrial ETC Redox Reactions
different affinities creates directionality
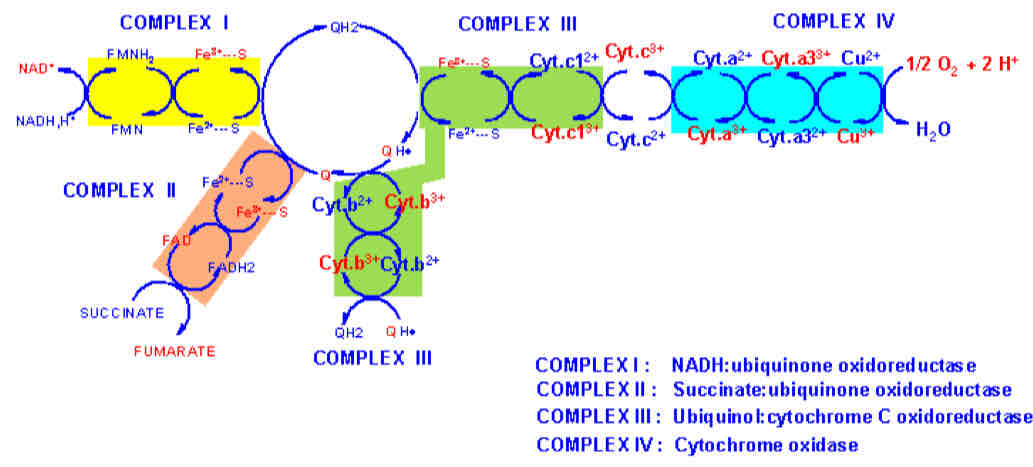
Redox Potential in ETC
redox potential increases as electrons flow down the respiratory chain to oxygen and can be directly related to the release of free energy
Direction of e- flow depends on affinity of different e- carriers
Affinity: expressed as a redox potential - the affinity of any pair of oxidized and reduced compounds (redox pair) for electrons
NADH has poor affinity for e- → very good electron donor
O2 has high affinity for e- → readily reduced, good electron acceptor
Direction of electron flow is from compounds with lower redox potential to compounds with successively higher redox potential (starting with NADH or FADH2 and ending with O2)
When electrons are passed from electron donor to acceptor, there is a release of free energy (potential energy has increased)
Free energy stored in electrochemical gradient is dissipated through ATP synthase to drive ADPphosphorylation
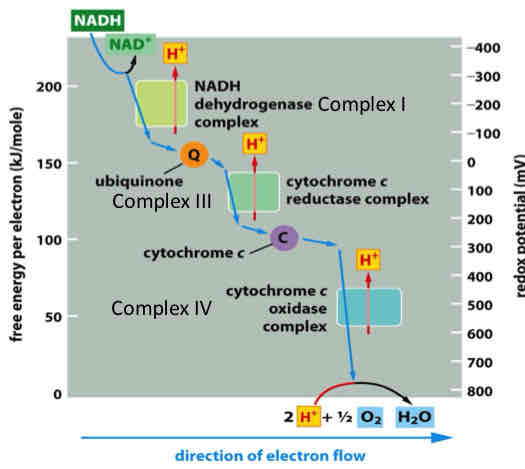
Chemiosmotic Energy Conservation
electrochemical gradient is dissipated through ATP synthase; free energy is used to drive ATP synthesis
Transmembrane electrochemical proton gradient is a form of energy storage that is adaptable to many forms of energy utilization
Protons go down concentration gradient through ATP synthase, driving reaction forward
Conservation of energy
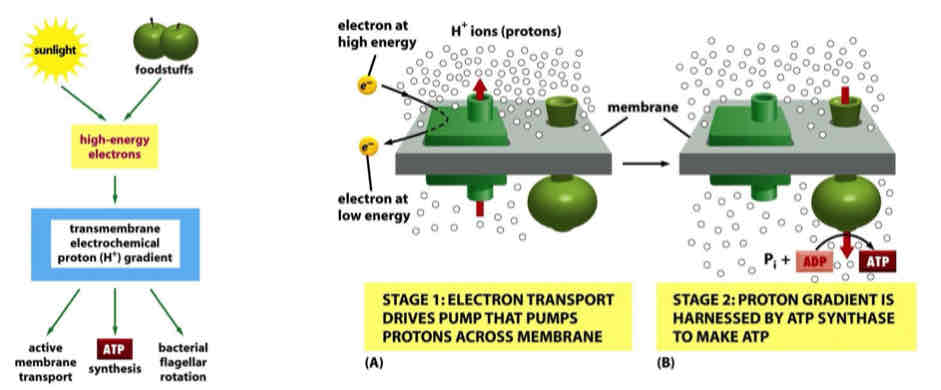
Processes in Mitochondria that use the Proton Motive Force
ATP synthase
Pyruvate transport by the MPC (pH gradient) (MPC: Mito Pyruvate Carrier)
Inorganic phosphate uptake by the PiC (pH gradient)
NA+ - H+ exchange (pH gradient)
K+ - H+ exchange (pH gradient)
Ca2+ uptake by the Ca2+ uniporter (membrane potential)
Protein import (membrane potential)
Transporters and ion channels utilize secondary active transport → use proton motive force
Each is a form of energy conservation
All the above processes occur across the IMM
Key Concepts of Energy Conservation by Chemiosmosis
Mitochondrial respiratory complexes are proton translocating redox enzyme systems
ATP synthase is a reversible proton pump
IMM have low proton conductance
Agents that increase proton conductance prevent energy conservation in mitochondria and uncouple substrate oxidation from phosphorylation (uncouplers)
IMM must contain transport proteins to permit the movement of adenine nucleotides, phosphate, and substrates and products of ox-red reactions in and out of mitochondria and to maintain osmotic stability (this costs some of the proton motive force)
Energy is Synthesized on Demand
take in food/fuel
Can store it or oxidize some of it
Ox couples to phos of ADP → ATP
Controlled by metabolic demand (ion pumps, heat, work)
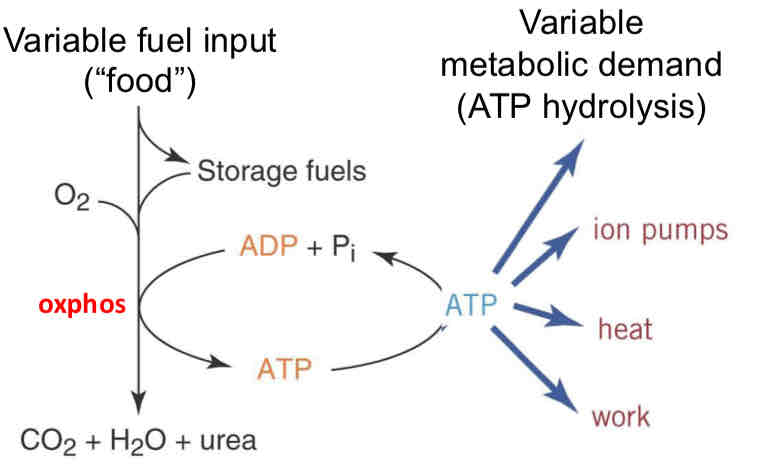
Respiratory Control
Electron transport driven by free energy available in electron carriers
Electron transport is restricted by chemiosmotic gradient: electron transport can only proceed when the gradient is dissipated (if hole in the bucket is plugged, eventually water can no longer be poured in)
In healthy mitochondria, electron transport keeps up with utilization of the energy stored in the gradient ie. with ATP hydrolysis leading to ADP transport into the mito matrix (more ADP = large hole size → faster water (electron transport) refilling)
The electron transport chain applies constant pressure to maintain an electrochemical gradient → adding ADP causes more electron flow
**Adding more electron transport chain and ATP synthase proteins will not necessarily increase ATP synthesis
need to have some release of gradient or oxidation will stop
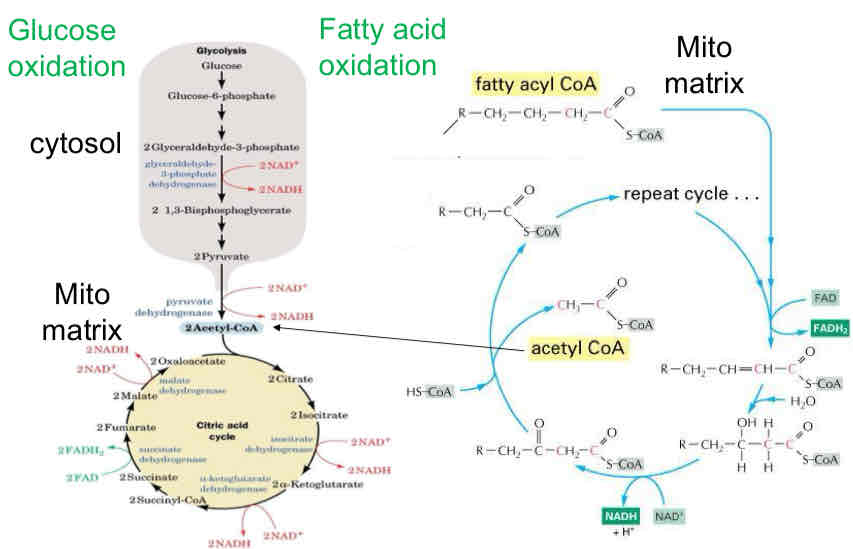
Where do NADH and FADH2 come from?
NADH → glucose oxidation (glycolysis and CAC) and fatty acid oxidation
FADH2 → succinate dehydrogenase (CAC) and fatty acid oxidation
Glucose and fatty acid oxidation
Glycolysis and citric acid cycle
Proton Leak and Uncoupling of Oxidative Phosphorylation
Uncoupling by lipid soluble weak acid, uncoupling protein (UCP), adenine nucleotide translocase promotes uncontrolled electron transport and hydrolysis of ATP
ANT exchanges ADP and ATP across the IMM, gets ADP into mito and ATP out → can dissipate electrochemical gradient
UCP also mediate leaks and dissipates proton gradient and generates heat
Weak acids (protonophors) can also penetrate the membrane and mediate leaks by carrying protons across membranes
More leaks → more electron flux → more pull on substrate/demand
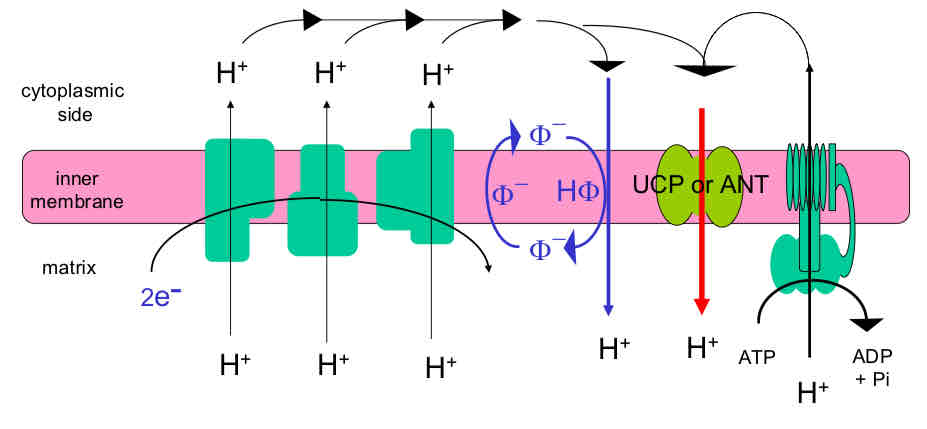
Uncoupling: Increasing Rate of Proton Leak through IMM
Some H+ permeability of IMM is essential for chemiosmotic energy conservation
Any factor that increases H+ leak rate will “uncouple” oxidation from phosphorylation
Uncoupling activates electron transport independent of availability of ADP and Pi (can promoter hydrolysis of ATP as a consequence of dissipating the proton electrochemical gradient)
Uncoupling proteins (UCP1) → membrane transporters that enhance H+ leak rate through IMM, but depend on binding of long chain FAs for activation
UCPs are regulated by binding of guanine or adenine nucleotides
UCP1 → only really good conductor of protons, only expressed in brown adipose fat (contains more mitochondria than white fat)
UCP1 turned on by hydrolysis of FA (from norepinephrine) → activates GPCR → increase in cAMP → activates PKA → lipolysis via HSL → TG broken down into FA → uncoupling → generates heat
ADT allows ADP entry into the matrix
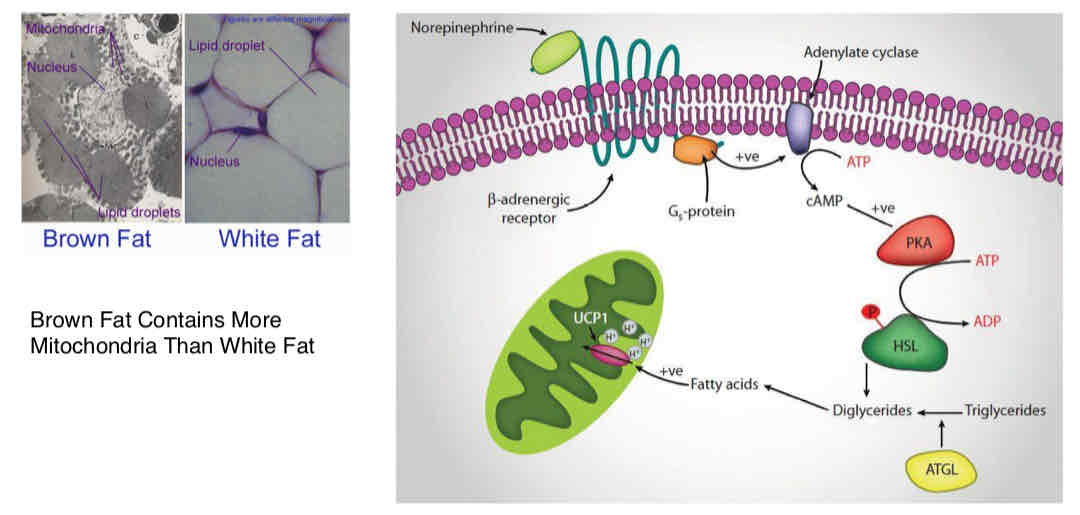
Benefits of Expressing Uncoupling Protein
Ensuring continue electron low → unrestricted substrate oxidation: promoting substrate removal unrestricted by the demand for metabolic energy
Oxidative stress defense suppression of excessive mitochondrial formation of ROS (occurs via glut of reducing power)
Thermogenesis: converting oxidative energy into heat
Efficiency: Organization of OxPhos Complexes
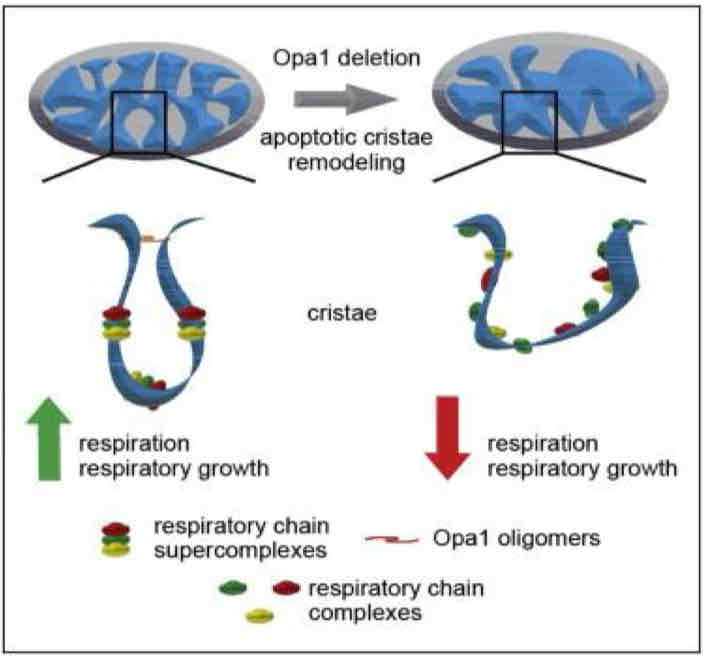
Oxidative Phosphorylaion occurs in the cristae space
MICOS Complex creates narrowing of cristae junction creating barrier for diffusion (diffusion limitation) → protons and cytochrome c cannot leave
Cytochrome C released when junction is disrupted → signal for apoptosis
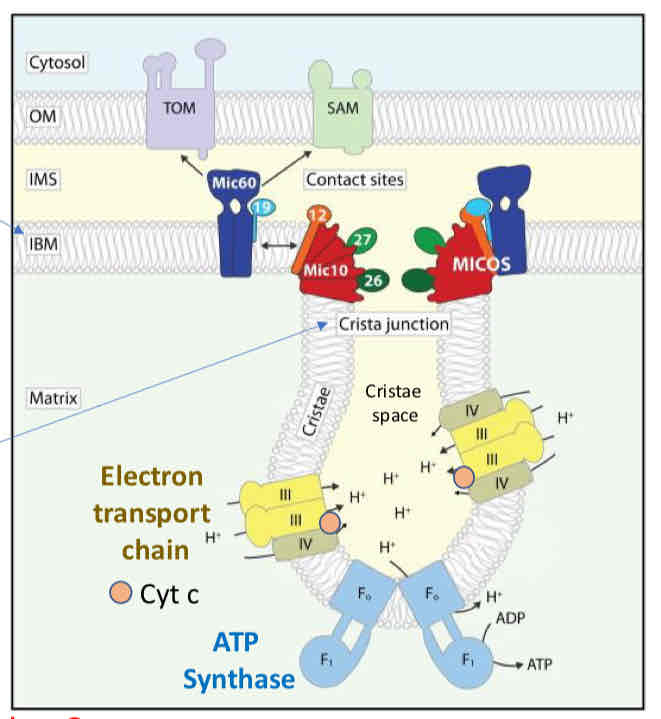
Respiratory Supercomplexes
ETC complexes in intact mitochondria exist as loose aggregates along the crista wall → super complexes or respirators
Respiratory chain super complex formation depends on the presence of supplementary subunits such as COX7A2- long/short and requires cardiolipin (IMM phospholipid)
Advantages relate to efficiency (through channeling of redox intermediates or association with other mitochondrial components) or regulation of activities of subsections of the respiratory chain
E- handed off more quickly/efficiently
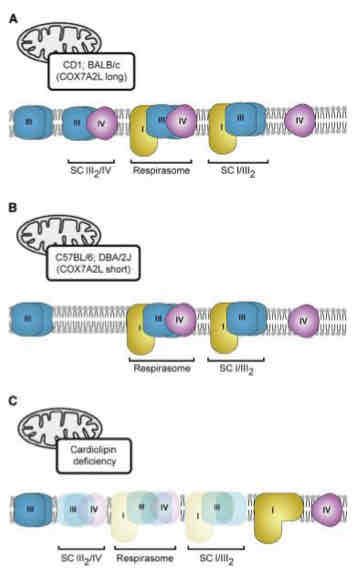
Disrupted Respiratory Chain Supercomplexes
Disrupted when cristae junctions loosen
May facilitate ROS formation and cytochrome c release due to electron leakage