Oceanology Exam 1
1/154
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
155 Terms
Science
the study of the natural world
Fact
an observable truth
Theory
an idea that is known to be true, but may not be testable or fully understood
Law
an idea that is known to be true that can be tested
Pseudoscience
a scientific sounding claim without support
Pseudo
meaning false
Paranormal
events that occur beyond the normal scope of scientific understanding
Para
meaning above
What is the first step of the scientific method?
Observe
What is the second step of the scientific method?
Identify a Problem
What is the third step of the scientific method?
Form a Hypothesis
What is the fourth step of the scientific method?
Test the Hypothesis
What is the fifth and final step of the scientific method?
Results/Conclusions
Distance
the amount of space between two points
Area
the amount of space on a surface
Volume
the amount of space within an object
Time
how far apart two events occur
Mass
how much matter is in an object
Speed
amount of distance traveled relative to the time travel
Velocity
is speed with direction specified
Acceleration
measure of speed changed (decreasing or increasing)
Force
push or pull on an object
Weight
amount of gravitational force acting on an object
Work
force applied across a certain distance
Power
work performed with time taken into account
Energy
the ability to preform work
Kinetic Energy
the energy in a moving object
Potential Energy
the energy that is stored in an object and can be released if certain conditions are at rest
Chemical Energy
energy absorbed in chemical reactions
Atomic Energy
vibration energy in every atom
Electromagnetic Energy
a spectrum of wave energy with different frequencies
Wavelength
the distance between two corresponding points of two consecutive waves
Frequency
a measure of how much energy a wave has
Electricity
the movement of electrons
Atom
smallest form of matter that has the property of elements
What is the charge of a proton?
positive
What is the charge of a neutron?
neutral or no charge
What is the charge of an electron?
negative
Atomic Number
the number of protons in the nucleus of the atom, how elements are defined
Atomic Mass
the sum of protons and neutrons
Atomic Charge
the electrons which revolve on shells around the nucleus of the atoms.
Formula of Atomic Number
P (protons)
Formula of Atomic Mass
P + N (protons + neutrons)
Formula of Atomic Charge
P - E (protons - electrons)
How many electrons can be held on the first shell of an atom?
two electrons
How many electrons can be held on the following shells after the first?
eight electrons
Isotopes
same element different masses
Radioactivity
the emission of energy, sometimes accompanies by particles, by an unstable atom trying to become more stable
Why would an atom become unstable?
there are too many protons and neutrons in the nucleus and not enough energy to maintain stability
What does it mean when an atom “fails” due to instability?
the atom is decaying at a measurable time, or half-life, by emitting radiation
How does an unstable atom become stable?
it goes through a process of emitting radiation which can happen quickly or take billions of years
What are the three types of radiation an unstable atom may go through?
alpha radiation, beta radiation, and gamma radiation
What is Alpha Radiation?
the atom kicks out a cluster of two protons and neutrons, or an helium atom
What is the size and speed of Alpha Radiation?
Large, slow
What is Beta Radiation?
when a neutron spontaneously breaks down and become a proton and an electron, since electrons can’t reside in the nucleus the atom will shoot it out
What is the size and speed of Beta Radiation?
small, fast
What is Gamma Radiation?
released energy in different wavelength frequencies
What is the speed and size of Gamma Radiation?
speed of light and no size because its a burst of energy
Half - Life
the amount of time it takes for half of the current number of parent atoms to decay into daughter atoms
Parent Isotope
the “before” or an unstable atom that will undergo radioactivity
Daughter Isotope
the “after” or the more stable product of the atom after undergoing radiation
Decay Train
the series of events that occurs when the daughter nucleus is unstable and continuously creates more daughter isotopes until the atom is stable
How do scientists use half-lives to determine the ages of elements?
Since half-lives don’t change, scientists measure the proportion of original radioactive isotope to the decay product to see how many half-lives have passed
Radiometric Dating
using radioactive isotopes and their half-lives to determine ages
Ion
a charged particle (atoms can gain or lose electrons which form charged particles)
Ionic Bond
opposite and equal charges are drawn to each other and stick
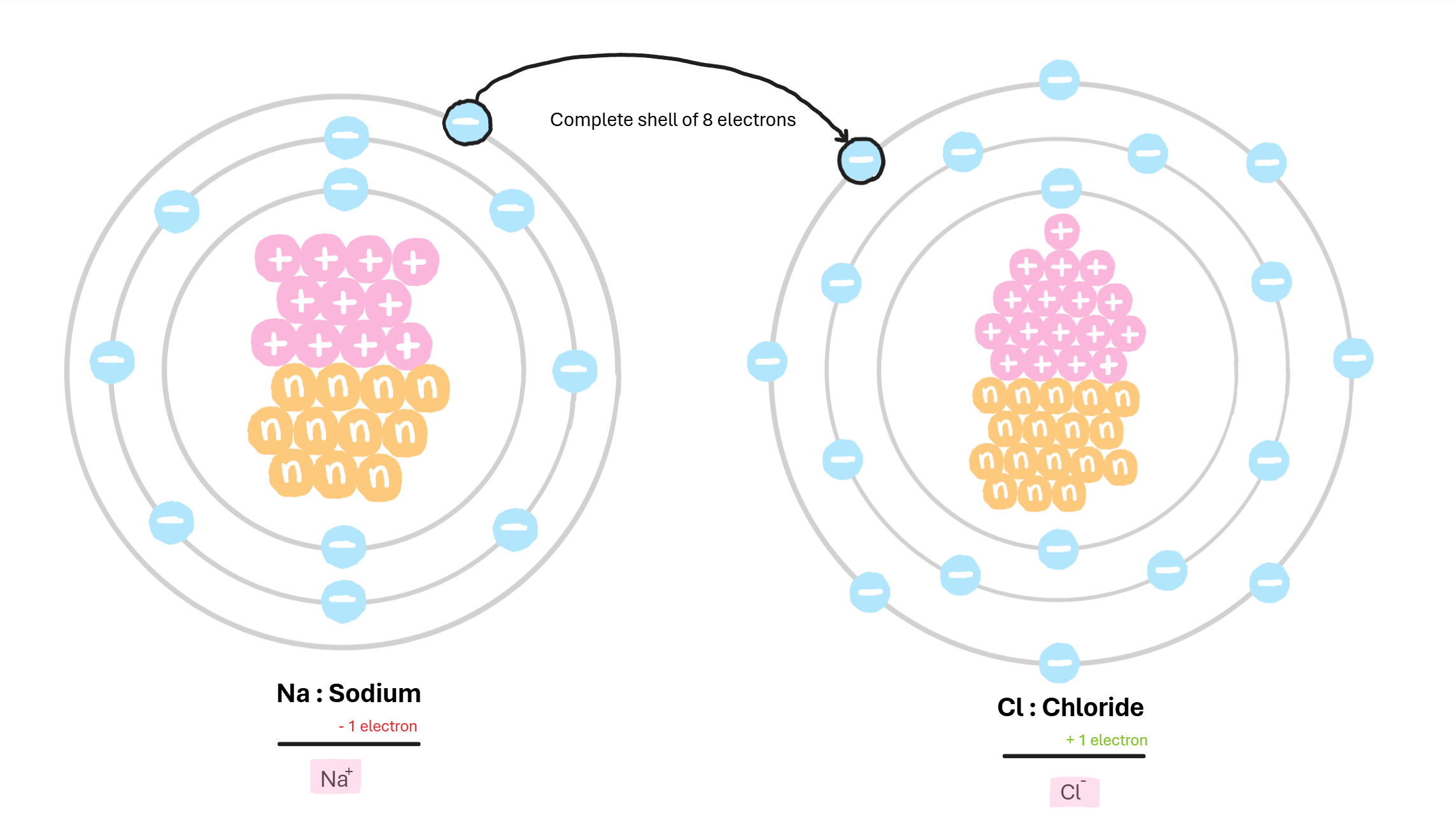
Valance
the outer most shell of the atom, that forms bonds with other atoms
Covalent Bond
two atoms share their electrons
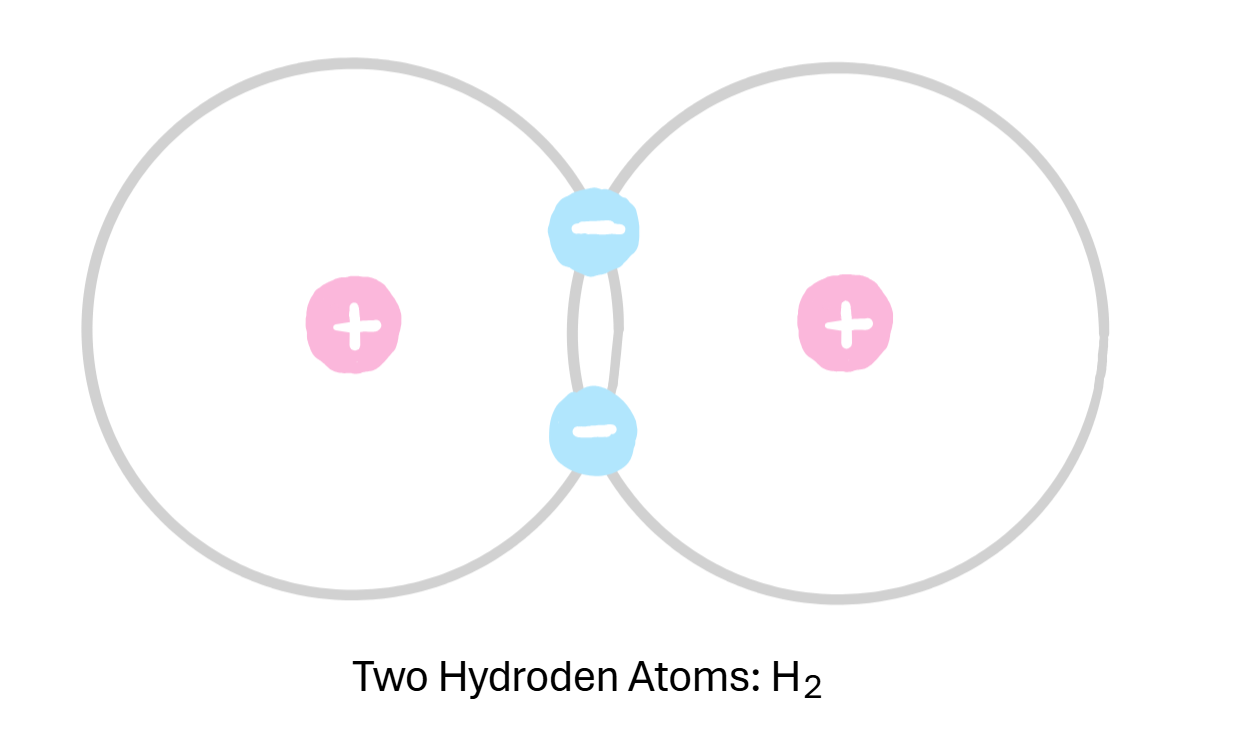
What can covalent bonds lead to?
diatomic elements or double atoms
Metallic Bond
a bond formed exclusive to metals, where all electrons are shared
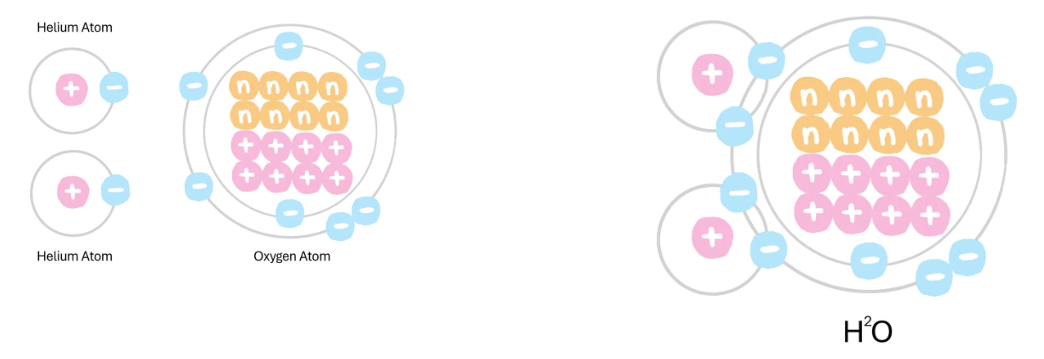
Draw how helium and oxygen bond? What bond is this?
Covalent Bond
Polarity
a molecule that has positive and negative sides
Why is water a great solvent?
Because of waters polarity it will pick apart substances with positive and negative particles as water is charged with both
How does water have surface tension?
Because of water molecules polarity, hydrogen bonds will form between them. In addition, since there is no force pulling on the molecules on the surface, they are able to bunch closer and tighter together.
Adhesion
water sticking to other materials
Cohesion
water sticking to itself
Heat Capacity
how much heat a material can gain or lose before it changes temperature
Does water have a high heat capacity?
Yes, water can absorb a lot of heat before the temperature changes.
Chemical Layers
defined by composition
Physical Layers
defined by what specific form each layer holds (liquid, solid, or in between)
What are the chemical layers?
crust, mantle, and core
What is the crust made of?
igneous, sedimentary, and metamorphic rocks
What is the mantle made of?
denser igneous rocks
What is the core made of?
iron and nickel
What are the physical layers?
lithosphere, asthenosphere, mesosphere, liquid outer core, and solid inner core
What is the form of the lithosphere?
it’s brittle or hard but liable to shatter or break easily
What is the form of the asthenosphere?
it’s ductile or able to be deformed without loosing toughness
What is the form of the mesosphere?
it’s ductile or able to be deformed without losing toughness BUT is denser and therefore more resistant to deformation
What is the form of the outer core?
it’s a liquid because it’s under the perfect amount of pressure and heat to move like a liquid
What is the form of the inner core?
it’s a solid because it’s under so much pressure and heat it makes it a solid
Draw the chemical layers and physical layers of the earth in comparision.
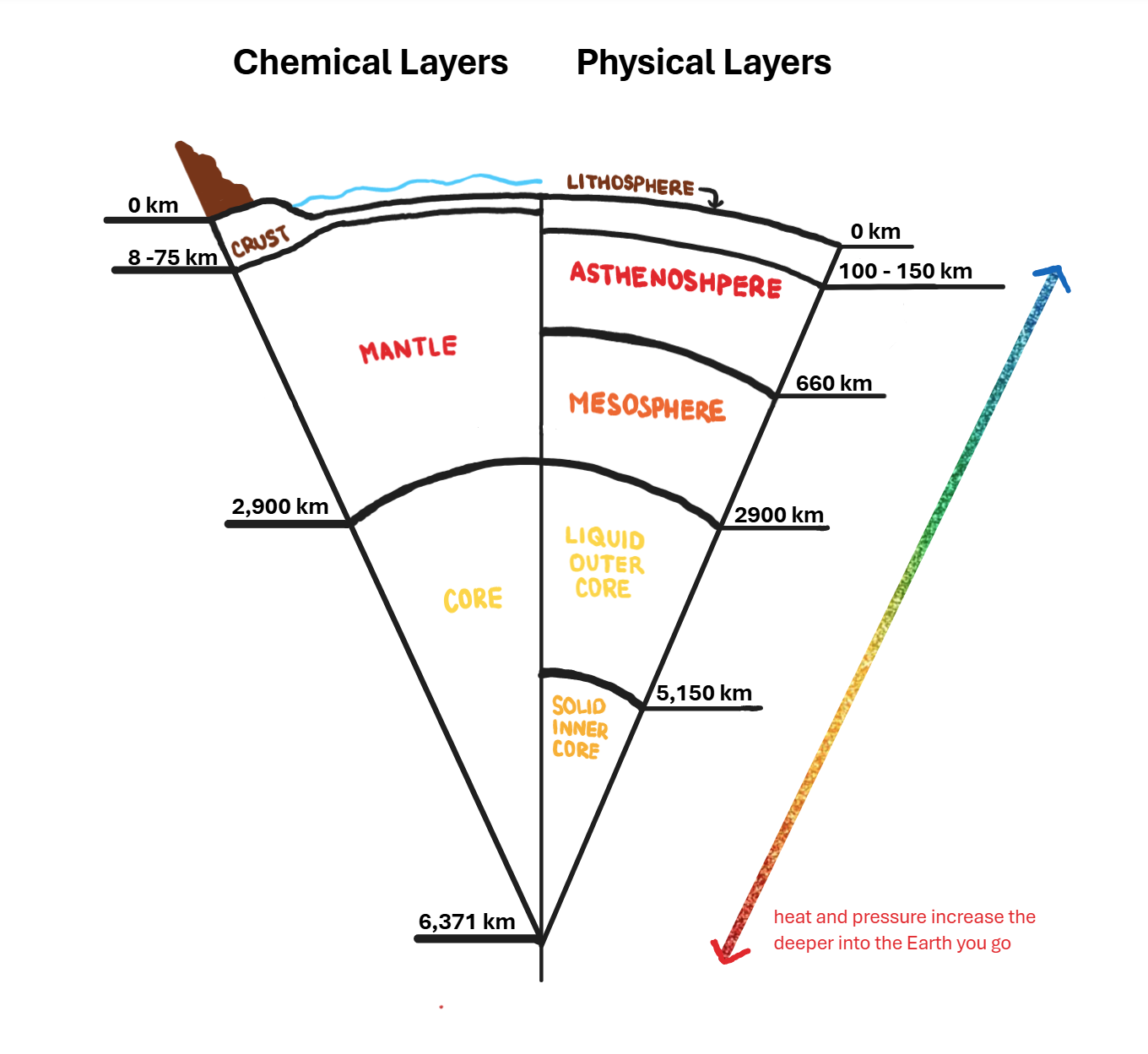
How does the Earth have a magnetic field?
The circulating convection currents of molten iron and nickel from the outer core generates electric currents which creates this magnetosphere
Theory of Plate Tectonics
states the outer most physical layer of Earth, or lithosphere, is broken each of which move independently
Boundaries
the contact or movement of tectonic plates
What are the three types of boundaries?
convergent, divergent, and transform boundaries
What are the different types of convergent boundaries?
Oceanic-oceanic, oceanic-continental, and continental-continental convergence
Subduction
one plate moves underneath another
Subduction Zone
the area where one tectonic plate moves under another
Draw an oceanic-oceanic convergence.
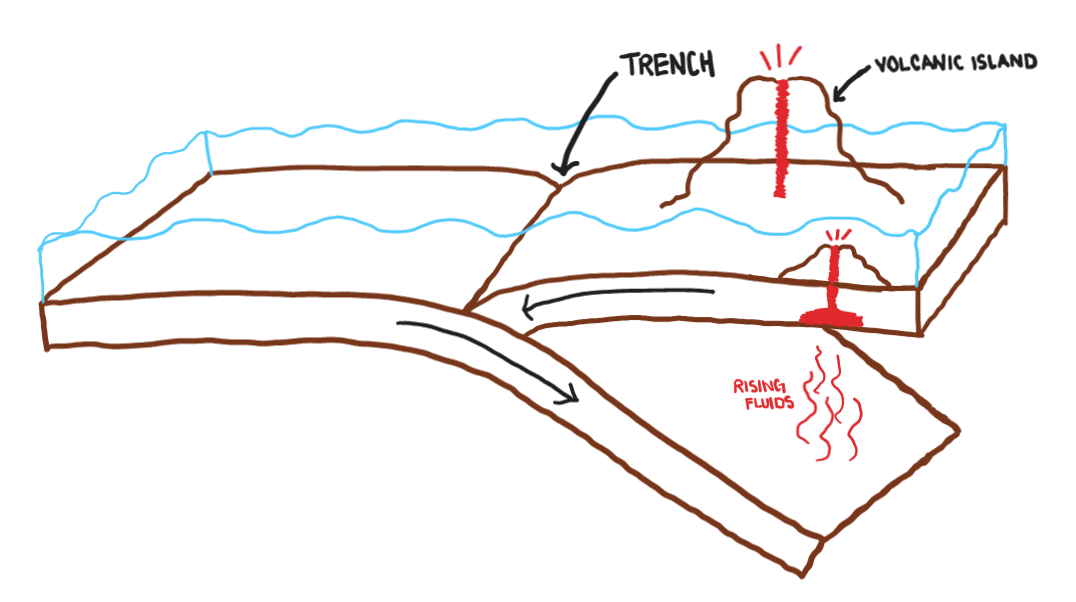
Explain an oceanic-oceanic convergence and each features that occur because of this convergence.
One plate will subduct under the other enter the asthenosphere. As the subducted plate continues to constantly move the fluids absorbed from the asthenosphere get released and migrate up. This “cooks” the plate above it causing liquid rock or magma to form and rise all the way to the surface. This is then released on the surface via a volcano, which is why near every subduction zone there is a volcano arc or multiple volcanic islands. In addition, the subduction created a trench on the ocean floor.