Chemical Reactions and Energy
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
What is the activation energy?
The miniumum amount of energy needed to start a reaction
2
New cards
Describe an exothermic reaction
Exothermic reactions release energy to the surroundings (increasing temperature).
3
New cards
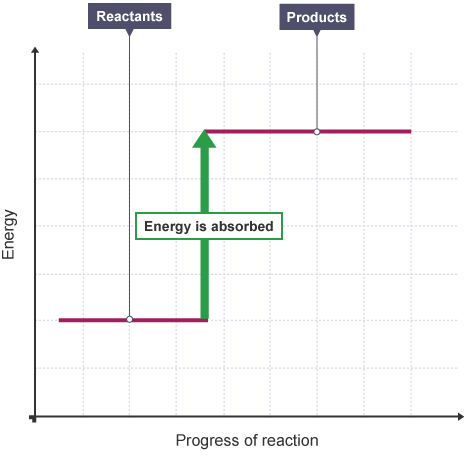
Describe an endothermic reaction
Endothermic reactions absorb energy from the surroundings (decreasing temperature).
4
New cards
What is an exothermic reaction profile look like?
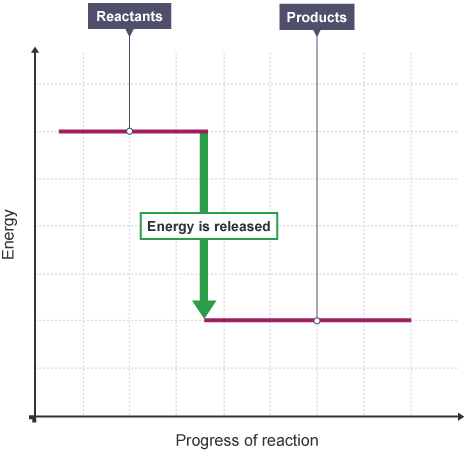
5
New cards
What is an endothermic reaction profile look like?