MCAT Biological and Biochemical Foundations of Living Systems
1/103
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
104 Terms
Digestive enzyme
Break down nutrients in food into small pieces that can be readily absorbed
Transport
Example: Hemoglobin
Carry substances throughout the body in blood or lymph
Structure
Example: Actin, Tubulin, Keratin
Build different structures, like the cytoskeleton
Hormone signaling
Example: Insulin, Glucagon
Coordinate the activity of different body systems
Defense
Examples: Antibodies
Protect the body from foreign pathogens
Contraction
Example: Myosin
Carry out muscle contraction
Storage
Example: Legume storage proteins, egg white (albumin)
Provide food for the early development of the embryo or the seedling
Metabolic enzymes
biological catalysts that facilitate biochemical reactions in the body, playing a crucial role in metabolism. These enzymes are typically proteins, and their activity can be influenced by factors such as temperature, pH, and concentration of substrates and products.
Hexokinase
Catalyzes the phosphorylation of glucose to glucose-6-phosphate, the first step in glycolysis. It plays a critical role in glucose metabolism and is regulated by the availability of glucose and ATP.
Phosphofructokinase-1 (PFK-1)
Often referred to as the “gatekeeper” of glycolysis, PFK-1 catalyzes the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate. It is a key regulatory step in glycolysis and is allosterically inhibited by ATP and citrate, while activated by AMP and fructose-2,6-bisphosphate.
Pyruvate Kinase
Catalyzes the final step of glycolysis, converting phosphoenolpyruvate (PEP) to pyruvate while producing ATP. It is regulated by fructose-1,6-bisphophate and is influenced by the energy state of the cell.
Lactose Dehydrogenase (LDH)
Catalyzes the conversion of pyruvate to lactate in anaerobic conditions and is important for regenerating NAD+ for glycolysis. It plays a role in lactic acid fermentation and is often measure in clinical settings to assess tissue hypoxia or damage.
Acetyl-CoA carboxylase (ACC)
Crucial for fatty acid synthesis, catalyzing the conversion of acetyl-CoA to malonyl-CoA. It is a key regulatory enzyme in lipid metabolism and is activated by citrate and insulin, while inhibited by palmitoyl-CoA.
Carnitine Acyltransferase I (CAT I)
Is involved in the transport of fatty acids into the mitochondria for β-oxidation. It catalyzes the conversion of acyl-CoA to acylcarnitine, allowing fatty acids to cross the mitochondrial membrane.
Glycogen Phosphorylase
Catalyzes the breakdown of glycogen to glucose-1-phosphate, playing a vital role in glycogenolysis. It is regulated by hormonal signals such as glucagon and epinephrine, which activate it during fasting or stress.
Hormones
Long-distance chemical signals released by endocrine cells (like the cells of the pituitary gland). They control specific physiological processes, such as growth, development, metabolism, and reproduction.
Protein-based hormones (Peptide Hormones)
Made of amino acids, are water soluble, and typically bind to receptors on the cell surface, triggering a cascade of reactions inside the cell.
Steroid-based hormones
Derived from cholesterol and are lipid-soluble, allowing them to easily pass through cell membranes and bind to intracellular receptors
Insulin
Important peptide hormone that helps regulate blood glucose levels. When blood glucose rises, specialized cells in the pancreas release insulin. The insulin binds to cells in the liver and other parts of the body, causing them to take up glucose. This process helps return blood sugar to its normal, resting lvls.
Denaturation
Changes in temperature and pH, as well as the presence of certain chemicals, may disrupt a protein’s shape and cause it to lose functionality.
Amino Acids
are the monomers that make up proteins. Physiological pH (7.2 - 7.4)
Polypeptide
is the chain that builds a protein. It usually a precursor to a protein. One or more polypeptides fold into a protein.
(ex. Letters (Amino Acids) -> Word (Polypeptide) -> Sentence (Protein))
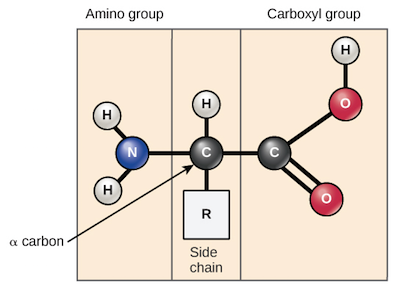
What do amino acids consist of?
alpha (α) carbon
bonded to an amino group (NH2), a carboxyl group (COOH), and a hydrogen atom.
Carboxyl group
is typically deprotonated and bears a negative charge; is a small cluster of atoms that makes a molecule acidic, its the defining feature of organic acids.
The key to this group is the O-H (oxygen-hydrogen) bond. It's a weak bond, so the hydrogen (H⁺) can easily pop off.
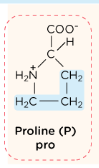
Proline side chain
is covalently bonded to the amino group present on the alpha carbon. This hyper-rigid ring structure creates kinks in the polypeptide structure.
Glycine
is the only non-chiral amino acid.
Serine, Threonine, and Tyrosine have what in common?
all have a side chain containing a hydroxyl (alcohol) group. This allows them to be phosphorylation targets or participate in hydrogen bonds.
Cysteine
contains a unique side chain in that it can create disulfide bridges with another cysteine when it is oxidized.
Histidine’s side chain has a pKa of what?
of 6, meaning unlike the other acids in this group, it is uncharged at a pH of 7.
Tyrosine’s side chain contains?
a polar hydroxyl group and a nonpolar aromatic ring. As a result, it has characteristics of both polar and nonpolar amino acids and can be categorized in either, depending on the criteria.
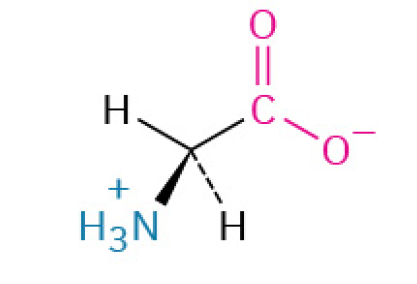
Glycine (Gly, G)
Simplest amino acid, with just a hydrogen atom as its side chain, providing flexibility to protein structures. It can be found on the inside or outside of proteins.
Nonpolar, Nonaromatic
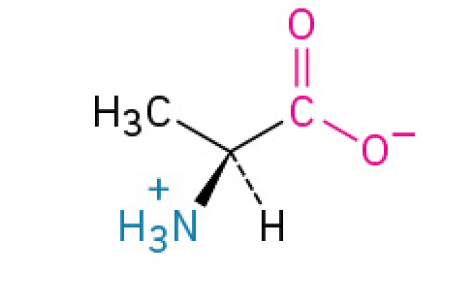
Alanine (Ala, A)
is a simple, versatile, and very common amino acid that the body uses for building proteins and for shuttling energy between your muscles and liver.
Nonpolar, Nonaromatic
Valine (Val, V)
is an essential building for making proteins. It’s known for being bulky and water-avoiding, which makes it important for creating the inner, structural “scaffolding” of proteins.
Your body cannot make it, you must get it from your food. used by muscles for energy.
Is one of the three branched-chain amino acids (BCAAs) Nonpolar, Nonaromatic
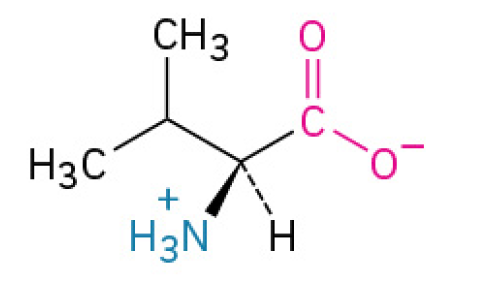
Leucine (Leu, L)
is an essential amino acid that does two jobs: it helps build the internal structure of proteins. And it acts as the primary signal to kick-start muscle growth and repair. BCAA also
Nonpolar, Nonaromatic
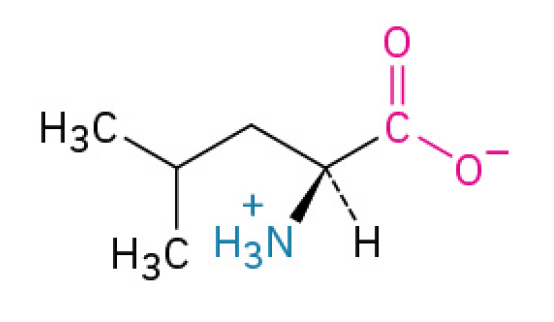
Isoleucine (Ile, I)
is an essential BCAA that specializes in energy production, blood sugar regulation, and overall metabolic balance, making it vital for endurance and recovery
Nonpolar, Nonaromatic
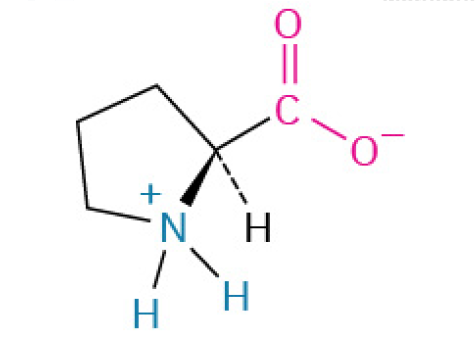
The 3 BCAA
Leucine: is the Leader who gives the orders to build
Valine: is the tank who provides strong defense and structure
Isoleucine: is the Scout who manages the energy supplies and keeps everything running smoothly in the background.
Methionine (Met, M)
contains a sulfur atom within a thioether group, and it’s often involved in initiating protein synthesis.
Nonpolar, Nonaromatic
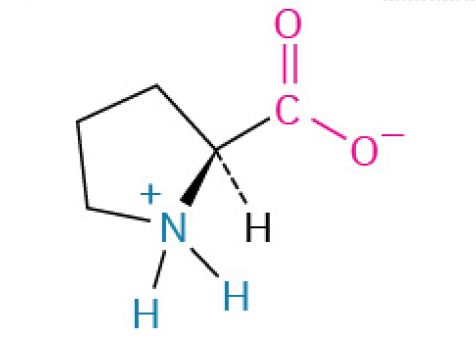
Proline (Pro, P)
is a unique amino acid that acts like a “hinge” or “kink” in protein chains. Its special shape is crucial for creating sharp bends and providing structural stability, especially in collagen (the protein that holds your body together)
Nonpolar, Nonaromatic
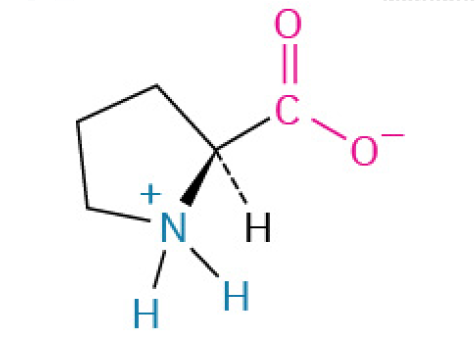
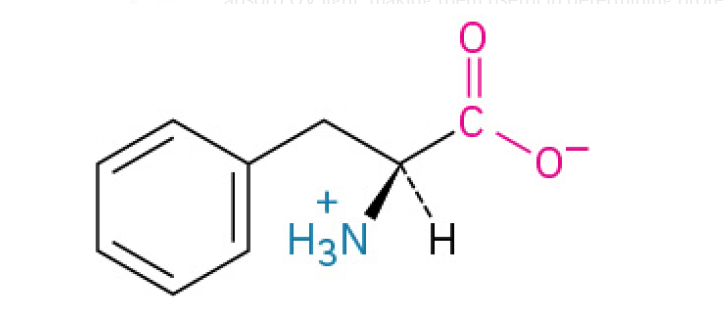
Phenylalanine (Phe, F)
is nonpolar, with a benzyl side chain, contributing to the hydrophobic core.
is an essential building block for proteins that is famous for being the starting material for many other important molecules in the body, including hormones and neurotransmitters.
The body uses this amino acid to create: Tyrosine, Dopamine & Norepinephrine (neurotransmitters that regulate mood, focus, and alertness), Epinephrine (Adrenaline), the “fight or flight” hormone. Thyroid hormones which regulate your metabolism.
Aromatic
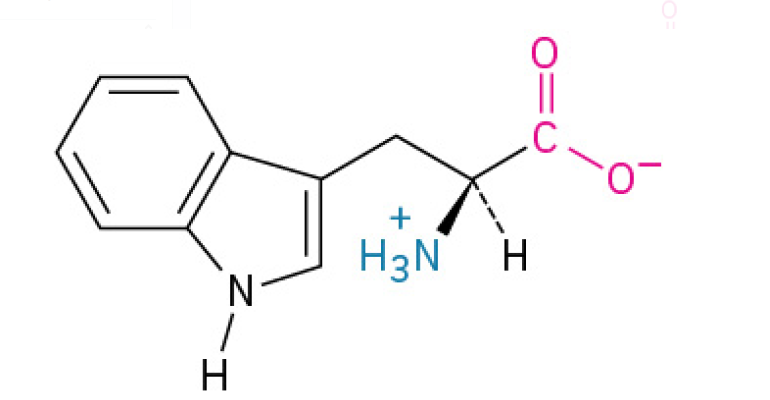
Tyrosine (Trp, W)
is the largest acid, with a complex double ring that includes a nitrogen atom, and plays an important role in protein-protein interactions.
is an essential amino acid that your body primarily uses to produce serotonin (for mood) and Melatonin (for sleep). Its the biological reason why a big meal can make you feel ready for a nap.
Aromatic
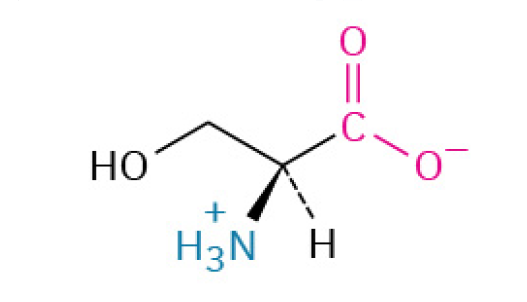
Serine (Ser, S)
is a water-loving, versatile amino acid that is essential for cell signaling (as an on/off switch), building cell membranes, and creating other crucial biological molecules.
Polar, Uncharged
Threonine (Thr, T)
is an essential amino acid that plays two critical roles: it acts as a molecular switch to control protein activity, and it provides structural support to tissues like skin, tendons, and teeth.
Like Serine, Threonine is crucial for a process called phosphorylation. A phosphate group can attach to its -OH group, which acts like a switch to turn other proteins on or off.
Polar, Uncharged
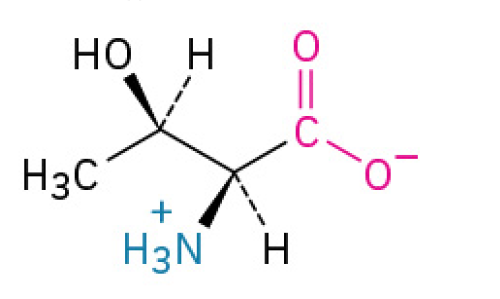
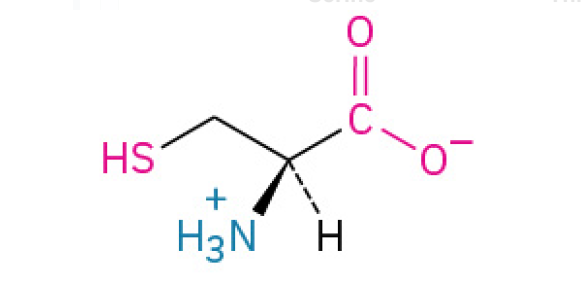
Cysteine (Cys, C)
is the amino acid that forms strong “disulfide bridges".” These bridges act like staples to lock proteins into their proper shapes, providing strength and stability to tissues like hair and skin, and ensuring proteins like hormones function correctly.
has a thiol group, making it reactive and prone to forming disulfide bonds, which are crucial for protein stability.
Polar, Uncharged
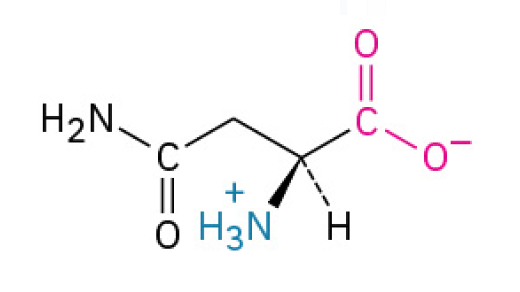
Asparagine (Asn, N)
is a hydrophilic, neutral amino acid that helps proteins stay soluble and serves as the main connection point for attaching sugar molecules, which are critical for protein function and cell communication. (non-essential amino acid; has a side chain that contains an amide group (a nitrogen and two hydrogens attached to a carbon)
Polar, Uncharged
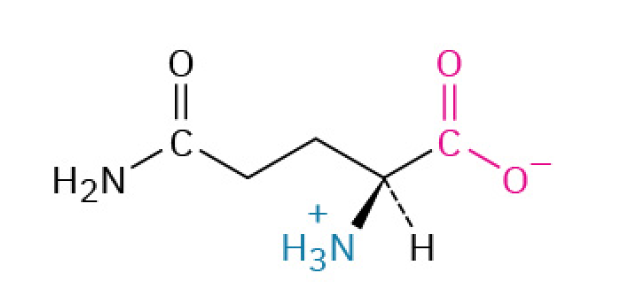
Glutamine (Gln, Q)
is a multi-purpose amino acid that primarily serves as a fuel source for your immune system and guy and as a safe transport molecule for nitrogen. It becomes especially important during times of physical stress.
It is the preferred fuel source for rapidly dividing cells, especially immune cells (like white blood cells) and the cells lining your intestines. This is why it's crucial for immune function and gut health.
Polar, Uncharged
Aspartic acid (Asp, D)
is a negative charged, acidic amino acid that primarily works to speed up chemical reactions in enzyme and acts as an excitatory signal in the nervous system
have carboxyl groups in their side chains, which lose protons and become negatively charged. They are often involved in active sites of enzymes.
Charged
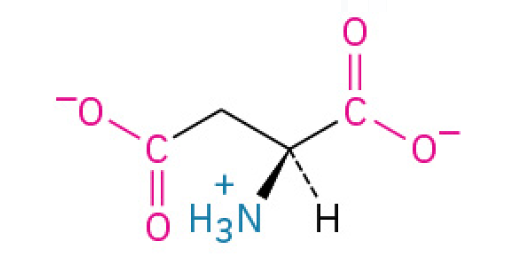
Glutamic Acid (Glu, E)
is a negatively charged amino acid that serves two primary purposes: It is the main excitatory neurotransmitter in your brain, essential for neutral communication, and it is the source of the savory umami taste in food (and its salt form, Monosodium Glutamate or MSG, is used as a flavor enhancer.)
have carboxyl groups in their side chains, which lose protons and become negatively charged. They are often involved in active sites of enzymes.
Charged
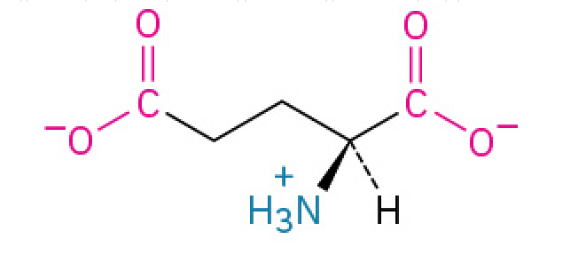
Lysine (Lys, K)
is an essential, positively charged amino acid that your body uses to stabilize proteins, support your immune system (especially against viruses like herpes simplex), and help you maintain strong bones by improving calcium absorption.
has a terminal primary amino group, often involved in binding to negatively charged molecules.
Charged
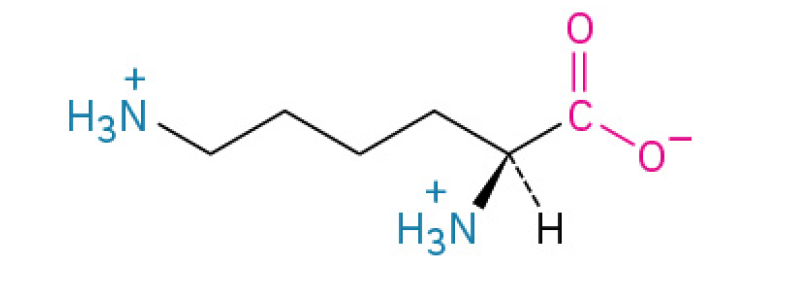
Arginine (Arg, R)
is vital amino acid primarily known for being the building block for nitric oxide, which improves blood flow, and for helping the body detoxify ammonia. This why it’s often associated with cardiovascular health, exercise performance, and healing. (Nitric Oxide – the "relaxation signal" that opens up blood vessels and improves circulation.)
contains a guanidinium group, making it highly basic and often involved in protein binding.
Charged
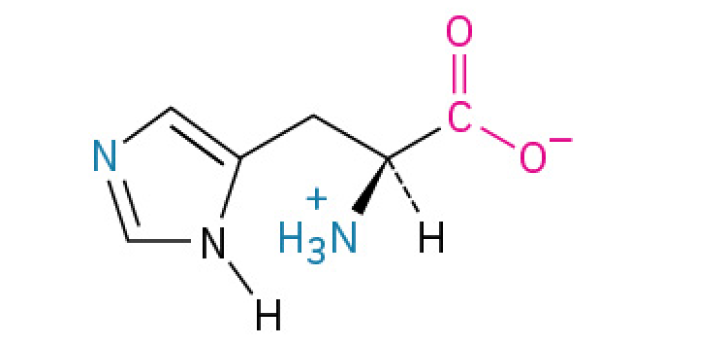
Histidine (His, H)
is an essential amino acid that functions as a molecular switch and buffer. It’s critical for enzyme function. maintaining pH balance, and is the building block for the important signaling molecule histamine. (Its special ability to grab or release hydrogen ions makes it crucial for controlling acid levels and enabling many biological reactions)
contains an imidazole ring, which can shuttle protons, playing a crucial role in enzyme active sites and buffering.
Charged
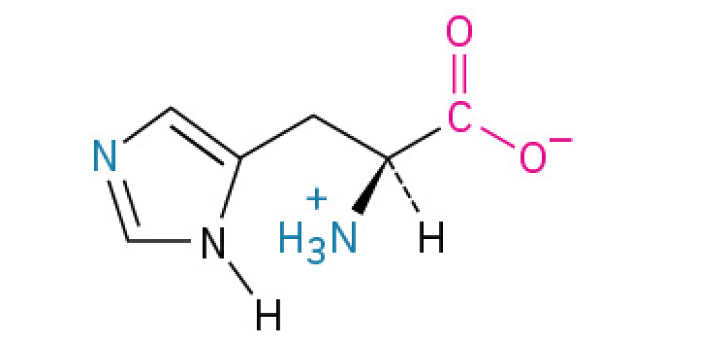
Essential Amino Acids
Cannot be synthesized by the human body and must be obtained from the diet. These include histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
Non-essential Amino Acids
Can be synthesized by the body. These include alanine, asparagine, aspartic acid, glutamic acid, serine, arginine, cysteine, glutamine, glycine, proline, and tyrosine.
Phosphorylation
Addition of phosphate groups (usually to serine, threonine, or tyrosine), playing a critical role in signaling pathways
Glycosylation
Attachment of carbohydrate groups, affecting protein folding, stability, and cell recognition.
Acetylation and Methylation
Modifications that typically occur on lysine residues, influencing gene expression and protein function.
Ubiquitination
Attachment of ubiquitin to lysine residues, tagging proteins for degradation.