AP Chemistry - 3.4
1/20
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What does Boyle’s Law state?
pressure of a gas is inversely proportional to its volume
As pressure increases, volume ____
decreases.
As pressure _, volume increases.
decreases
Formula for Boyle’s Law
P1V1=P2V2
What does Charles’ Law state?
Volume of a gas is directly proportional to its temperature
Formula for Charles’ Law

Celsius to Kelvin formula
C + 273=K
NOTE All temperatures should be in Kelvin
NOTE All temperatures should be in Kelvin
What does Guy Lussac’s Law state?
The pressure of a gas is directly proportional to its temperature.
As pressure increases, temperature _.
increases
As temperature decrease, pressure _.
decreases
Guy Lussac’s Law formula
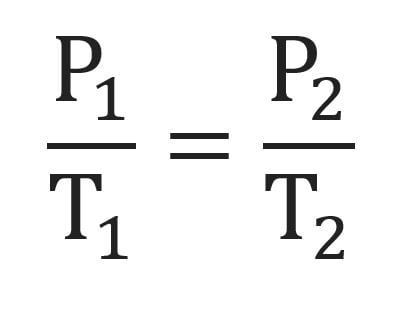
Combined gas law formula
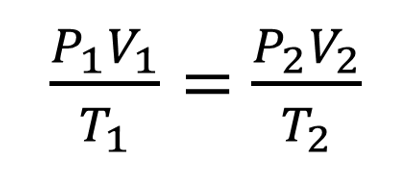
Ideal gas law
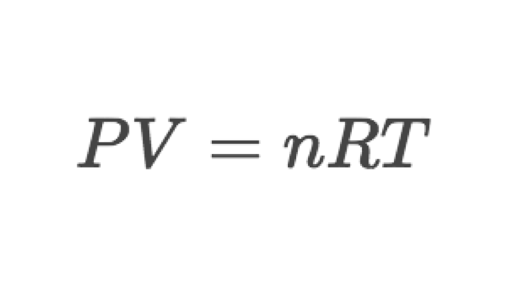
P
pressure(atmospheres)
V
volume(liters)
n
number of moles of gas
T
temperature (Kelvins)
R
Universal gas constant
Universal gas constant
0.0821
Dalton’s Law of Partial Pressures

