Preparation of a sample of Soap
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
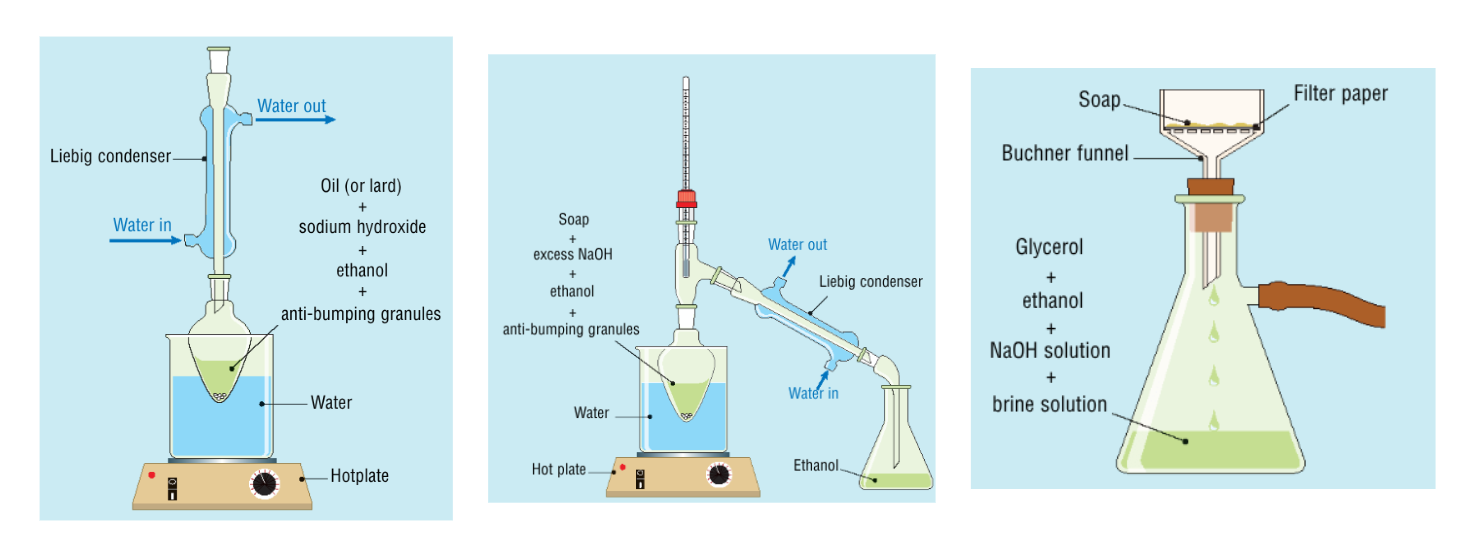
Key steps + observations in the procedure for the preparation of a sample of soap:
set up a reflux apparatus
oil containing glycerol tristearate, sodium hydroxide, ethanol and antibumping granules are added to a pear shaped flask
gyceryl tristearate is the key component for making soap
sodium hydroxide is the base present for base hydrolysis/saponification to occur
antibumping granules ensures a smooth, contolled boil
ethanol acts as a solvent for the oil
reflux for 30 minutes to ensure reaction completion and no loss of ethanol solvent
the walls of the coated become coated with the sodium salts of the fatty acids that have not been hydrolysed
set up distillation apparatus to collect ethanol, which has a lower boiling point
the contents of the pear shaped flask is poured into a beaker containing brine, which will precipitate the soap and dissolve excess sodium hydroxide
use a small amount of water to ensure all washings go into the brine solution, only a small amount to ensure the solution is still saturated
soap (sodium stearate) is filtered off and washed with ice cold water, to remove excess sodium hydroxide; the water being ice cold prevents the soaps from dissolving too quickly
soap is allowed to air dry overnight on filter paper
What is refluxing and why is is used
a lab technique in which a liquid is boiled in a container that is attached to a vertical condenser
vapour from the boiling liquid condenses and flows back into the flask which keeps the liquid boiling without the loss of vapour
this prevents the flask running dry whilst allowing sufficient time for the reaction to occur, maximising the yield
What is brine?
a concentrated solution of sodium chloride (NaCl or salt) in water
What is the test to confirm that soap has been produced?
put in a test tube with water, stopper and shake
the soap forms a lather in the presence of water
Write a word + chemical equation for the preparation of soap:
Glycerol tristearate + Sodium hydroxide → Sodium Stearate (Soap) + Glycerol
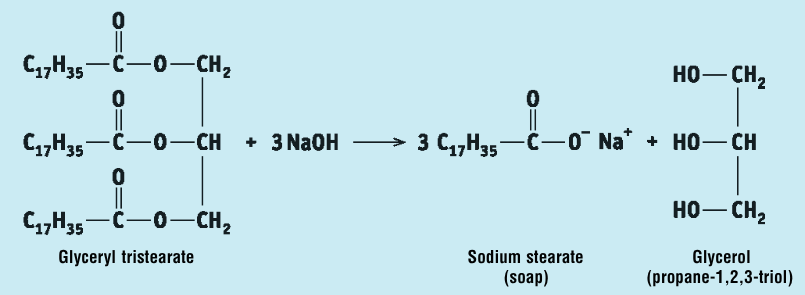
What is the IUPAC name for glycerol?
Propan-1,2,3-triol
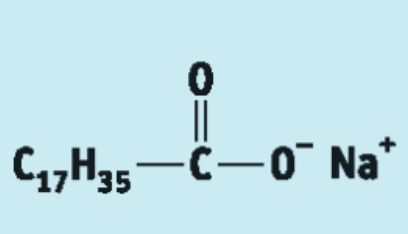
Draw the structure of sodium stearate:
sodium octadecanoate