Lecture 3 - Entropy
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Expression of first law of thermodynamics
Law = conservation of energy
Related to work ΔW and heat ΔQ exchanged with surroundings as ΔU = ΔW + ΔQ.
ΔU for ideal gas
ΔU = n* Cv*ΔT
ΔU for different processes
ΔU = Internal energy
For isothermal processes: ΔT = 0 → Internal energy = constant ΔU = 0 → ΔQ = -ΔW
For isochoric processes → no work → ΔW = 0 → ΔU = ΔQ
For adiabatic processes → no heat flow → ΔQ = 0 → ΔU = ΔW
Adiabatic processes
adiabatic process: ∆𝑄 = 0
System is adiabatically isolated (perfect insulation)
In real life → insulation ≠ perfect
Very fast processes → limited heat exchange → assume adiabatic → ∆𝑈 = ∆𝑊
Adiabatic cooling: decrease pressure → system expands → system performs work on surroundings (∆𝑊 < 0) → internal energy decreases → temperature decreases
∆𝑈 = n𝐶𝑣∆𝑇
Example adiabatic process (can of coke)
Opening a can of coke:
Can is openend, CO2 starts to expand.
Internal energy of the CO2 goes down, therefore temperature also goes down
Surrounding becomes cool, in surrounding we have moisture (water vapor)
Therefore release of water vapor from can happens
Would not happen in desert because there is no water vapor in the air.
Example of adiabatic process (Fire exthinguisher)
Fire extinguishers also are filled with CO2
The cans also have higher pressure
When using, liquid expands very fast, leading to very fast cooling
CO2 turns into solid, powder like CO2
Macrostate
Number of balls * level
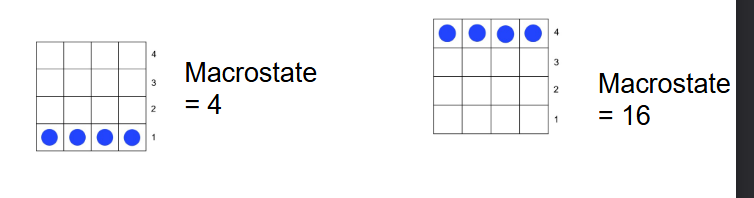
When is entropy highest?
At macrostate 10
Highest when system arranged in most probable way
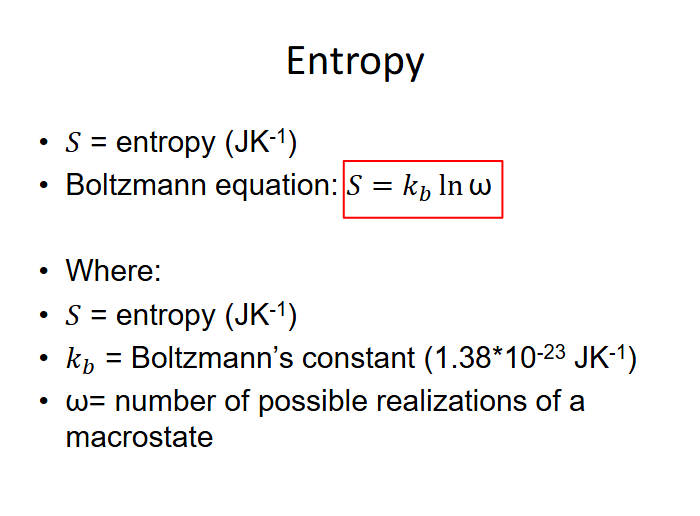
How to calculate entropy?
Shape of polymer and entropy
Pull on each end of polymer chain
Enough force → completely extend → less probable configuration → reduction in entropy
Release tension
Back to more probable configuration → increase in entropy → entropic elasticity
Not necessarily exactly same configuration as start
Enthalpy
Concept of enthalpy H = U + pV
H is a state variable (does not depend on the path)
ΔH = ΔQ
Heat Q is not a state variable
Gibbs free energy
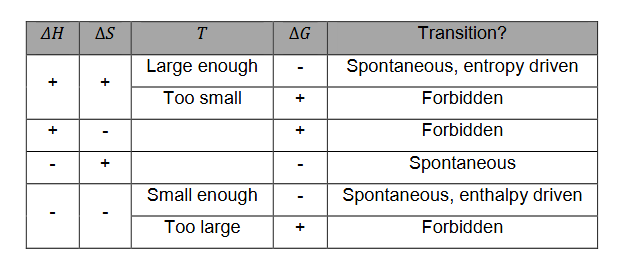
System will always strive to have a minimal value for G at fixed T and p
Gibbs free energy is minimized when enthalpy is minimized and entropy is maximized.

What happens when G is 0?
We have thermodynamic equilibrium
When we have a change in the system, G should decrease, otherwise the change is forbidden
G<0
Relation H, S, T and G
Melting of ice as an example of Gibbs free energy
Heat is added to induce phase transition → ΔH > 0
Water has a higher degree of disorder than ice ΔS > 0 → -TΔS < 0
At melting temperature or higher T: TΔS > ΔH → ΔG < 0 and the transition happens.
Chemical potential
A chemical potential μ to each phase
μsolid, μliquid, μgas
When in the liquid phase for example μliquid < μsolid, μgas
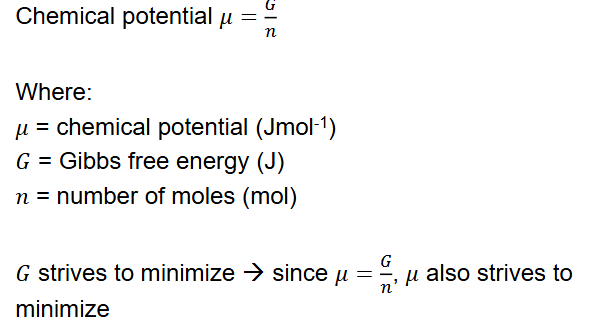
What is the most stable phase?

What are G and μ when you are on the border of a line in the phase diagram?
Because G is equal so will μ be
So at the triple point μsolid = μliquid = μgas
Relation between Δμ, ΔT and -S
When entropy is higher, the slope is also greater.
Using this formula you can predict the entropy for each state and model it.
Again when these phase lines intersect μ increases.
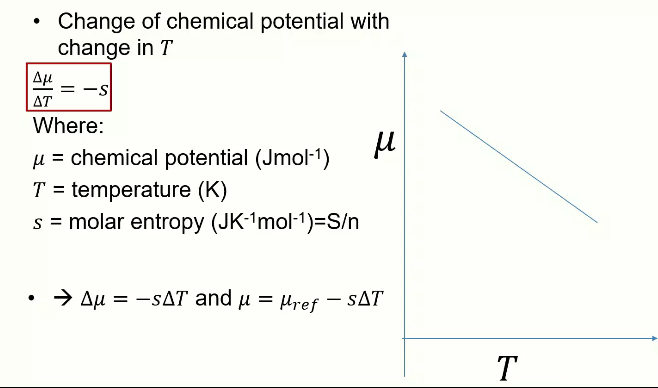
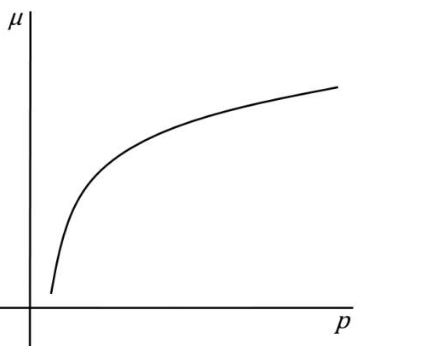
Relation between Δμ, Δp and v
Can also be used to make a line as a function of pressure
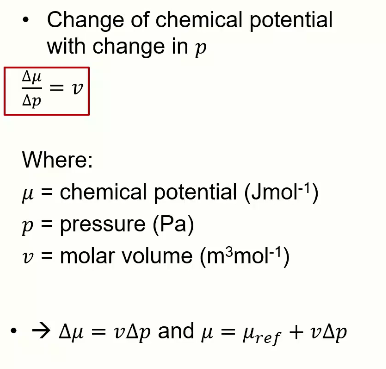
How to find the chemical potential of an ideal gas
Chemical potential = RT*ln(p2/p1)
In the case of a mixed gas:
Chemical potential = μref + RTlnxgas
What causes a change in W (in first law of thermodynamics)
Any change of space
Compression of gas: -W
Expansion of gas: +W
Change in W will be positive
Relation temperature and internal energy
Has a direct link: higher t = higher delta U
Graph of ideal gas molar volume against pressure
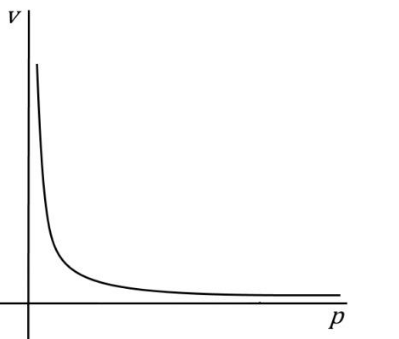
Graph of ideal gas chemical potential against pressure