Material Science Final
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
88 Terms
There have been many attempts to manufacture and market plastic bicycles. All have been too flexible. Which design-limiting property is insufficiently large?
Stiffness
Silver has a high embodied energy – its value is 1,500 Mj/kg. What does this mean? It means that the mining and beneficiation of the ore and its subsequent refining to make silver consumes …
1500 Mj of energy for each kg of silver produced.
Figure shows a cubic element of material with Young's modulus E and Poisson’s ratio that is subjected to normal stresses σ1, σ2 and σ3, resulting in strains ε1, ε2 and ε3. Find an expression for the dilatation (Δ) or fractional volume change, when a unit cube experiences strains of ε1, ε2 and ε3, assuming the strains are elastic and therefore small (<< 1)
(ε1 + ε2 + ε3)
What would be the units of force that the formula directly produces?
eV/nm
Which of the following configurations corresponds to an inert gas?
NoA
Regarding the spin of the electron?
It is an intrinsic property of the electron.
Which of the following quantum orbitals is not permitted?
3f
What type of bond is expected between two nitrogen molecules?
Fluctuating Induced Dipole Bonds
Which of the following properties of a materials is not determined by the electronic structure?
NoA
What type of bonding is expected among xenon atoms?
Fluctuating Induced Dipole Bonds
What type of bond is expected between two oxygen molecules?
Fluctuating Induced Dipole Bonds
What type of bond is expected among silver atoms?
Metallic Bonding
Aluminum has a high carbon footprint – its value is 13.1 kg/kg. What does this mean?
The global-warming equivalent of 13.1 kg of CO2 when 1 kg of aluminum is produced.
What is a fluctuating Induce dipole bond?
Interaction of an instantaneous dipole with the dipole it induces in an adjacent molecule or atom
Why did pipelines break during freezing? Because …
all of the above
Regarding the unit cell, indicate the incorrect sentence:
NoA
Which of the following does not correspond to Tin?
heat insulator
Natural rubber has a high-water demand – its value is 17,500 liters/kg. What does this mean?
The process to extract 1 kg of rubber from trees and its subsequent process to shape it demands 17,500 liters of water.
Which of the following is the unit cell of diamond?
Diamond cubic
Figure shows a cubic element of material with Young's modulus E and Poisson’s ratio that is subjected to normal stresses σ1, σ2 and σ3, resulting in strains ε1, ε2 and ε3. Find an expression for the dilatation (Δ) or fractional volume change, when a unit cube experiences strains of ε1, ε2 and ε3, assuming the strains are elastic and therefore small (<< 1).
NoA
For what value of Poisson’s ratio is volume conserved?
E/2
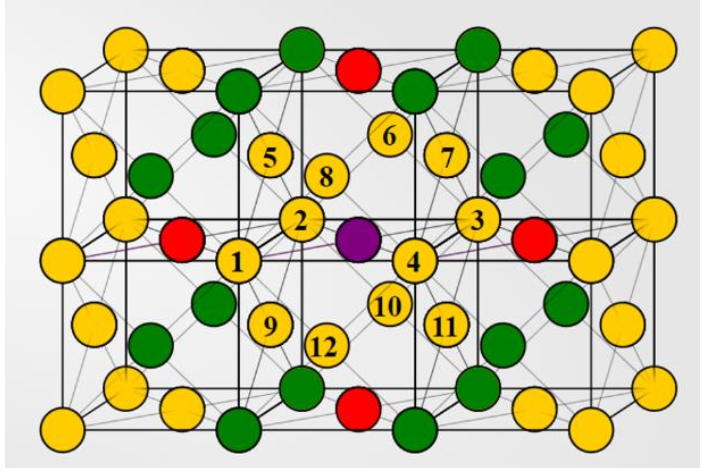
Indicate the number of next nearest neighbors.
6
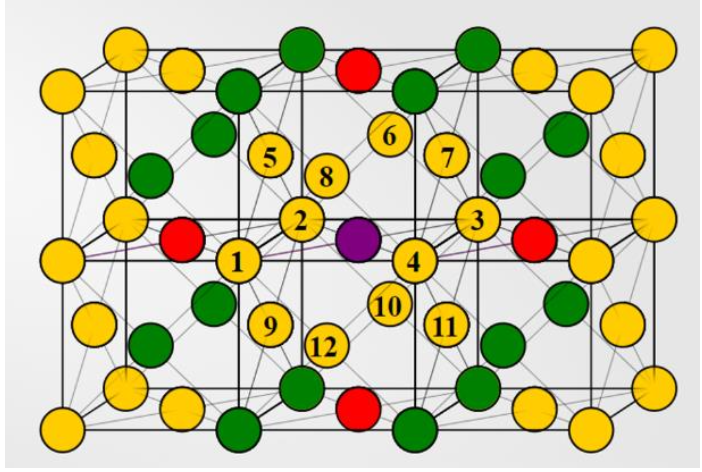
Indicate the number of next next nearest neighbors.
24
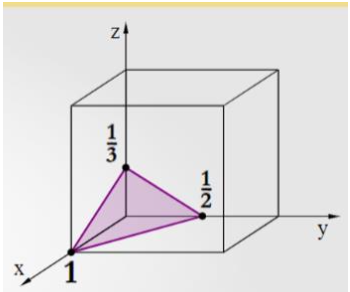
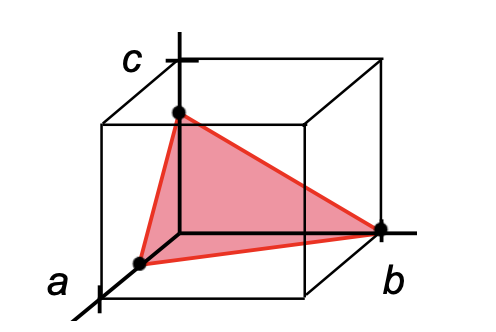
Indicate the Miller indices of the plane shown in the figure (right side).
*They are the reciprocals of the shown indices - if still a fraction multiply out
(1,2,3)
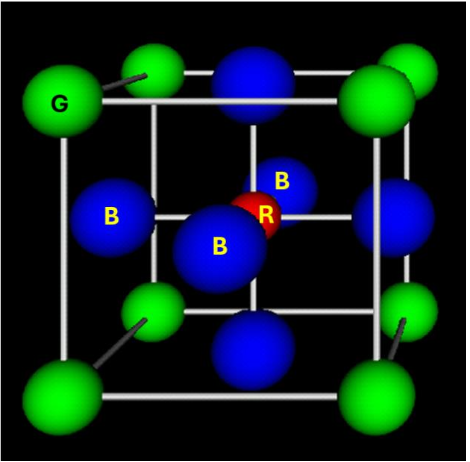
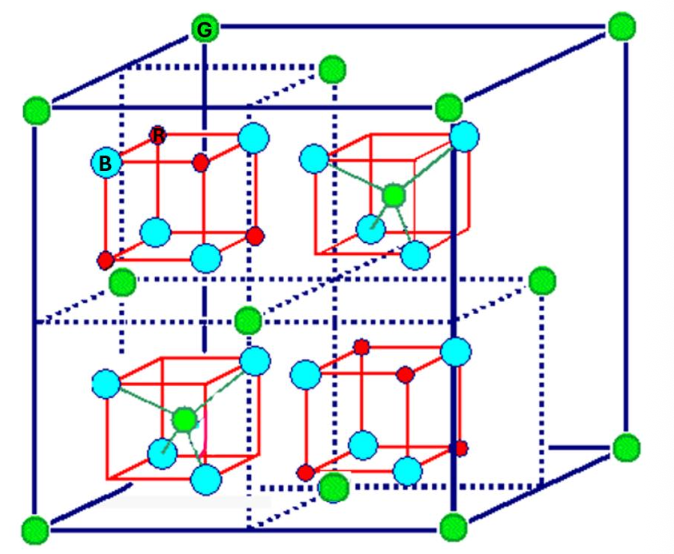
Indicate the color of the negative ions in the perovskite structure.
Blue
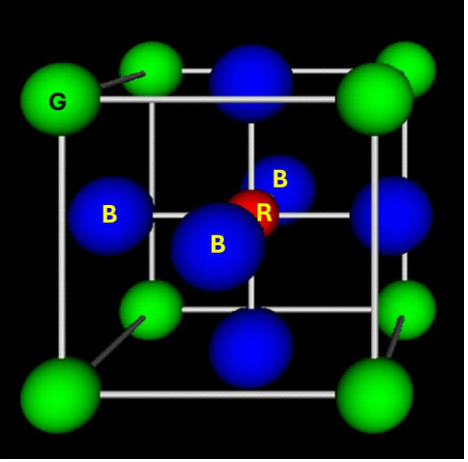
How many NN has a red atom of the perovskite?
6
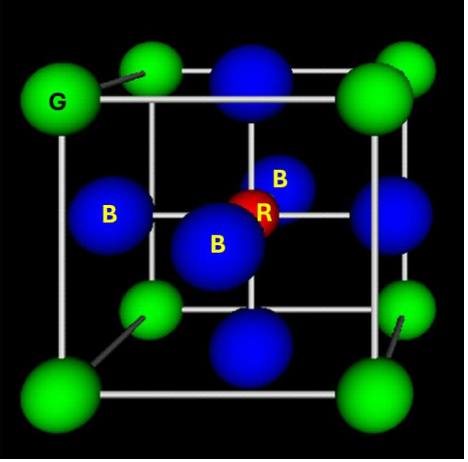
How many NN has a green atom of the perovskite?
12
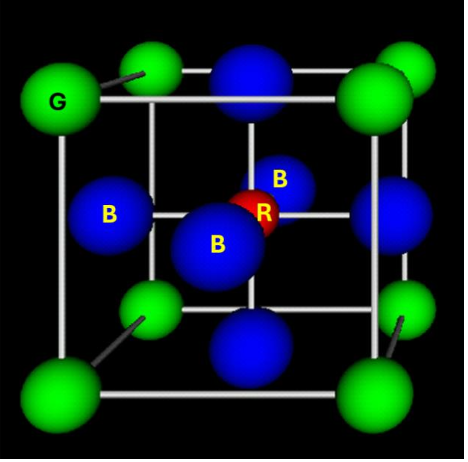
How many NN has a blue atom of the perovskite?
2
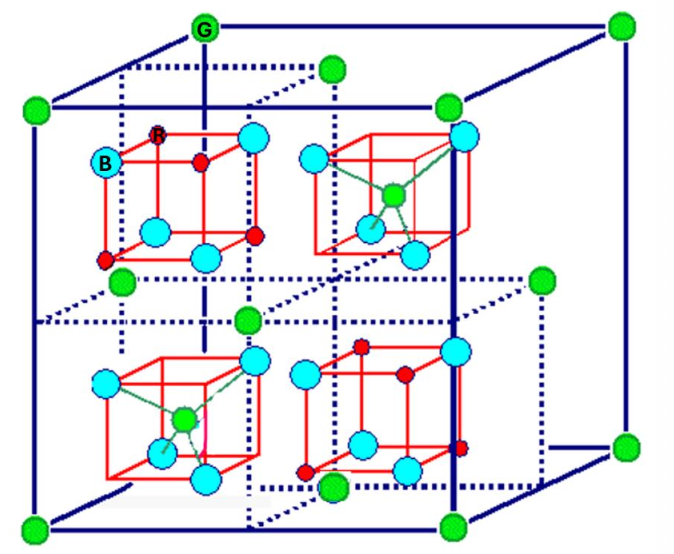
For the spinel structure, indicate the number of green atoms in the unit cell.
8
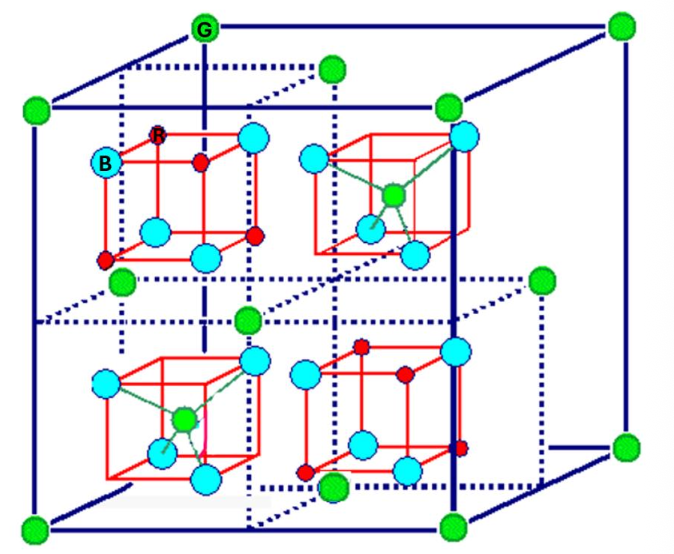
For the spinel structure, indicate the number of blue atoms.
32
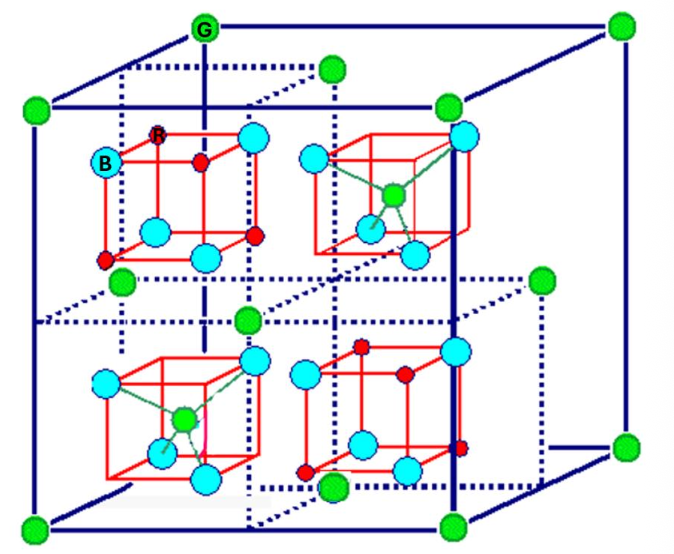
For the spinel structure, indicate the number of red atoms.
16
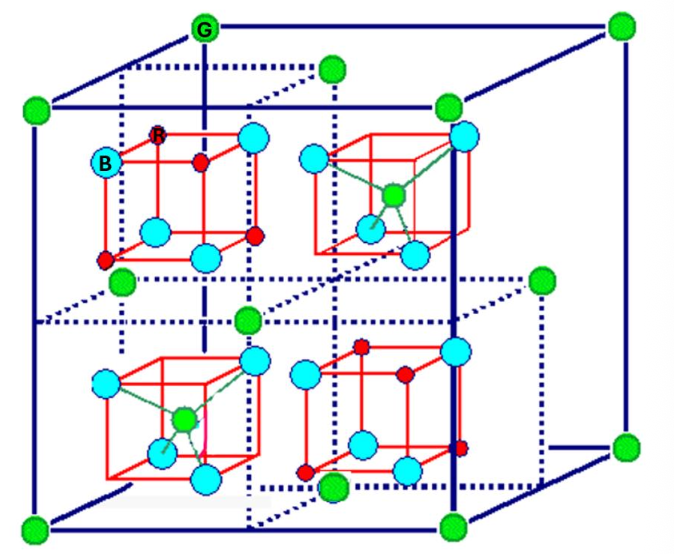
For the spinel structure, indicate the anion.
blue
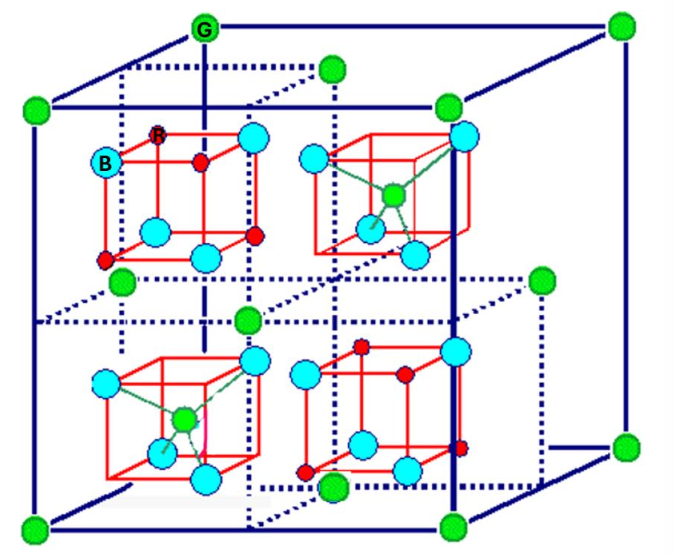
For the spinel structure, how many NN has the green atom?
4

For the spinel structure, how many NN has the red atom?
6
For the spinel structure, how many NN has the blue atom?
4
What plane is the shaded one? (miller indices)
(6 3 4)
![<p>Which of the following directions is<span style="line-height: normal;"><span> perpendicular to the [100]</span></span></p>](https://knowt-user-attachments.s3.amazonaws.com/da39524b-3d27-4a39-9623-2d9ab69a88e8.png)
Which of the following directions is perpendicular to the [100]
[1 0 0]
![<p>Which of the following directions is<span style="line-height: normal;"><span> perpendicular to the [111]</span></span></p>](https://knowt-user-attachments.s3.amazonaws.com/8b4323ba-278c-4786-8b35-e80398ec90c0.png)
Which of the following directions is perpendicular to the [111]
[1 1 1]
Battery grade lithium metal has an embodied energy of 40 kWh/kg. What does this mean? It means that the mining and beneifcation and its subsequent refining to make lithium ready for battery manufacture consumes…
NoA
A cube of linear elastic material has vertical compressive stress in one direction and constrained in the other two, find the expression for the induced transverse stress.
(stress 1/strain 1) = E*(1-v)/(1-v+2v²)
Which expression is correct?
e2 =e3 = 0
What type of bonding would you expect for Sr?
Metallic
What type of bonding would you expect for MgI2?
Ionic
What type of bonding would you expect for AlP?
Covalent
What type of bonding would you expect for Diamond?
Covalent
When water freezes, which of the following statements is false?
Frozen water does not have hydrogen bonding; however liquid water does
Rank the bonds Na-Cl, Li-H, H-C, H-F, and Rb-O in order from the most covalent to the most ionic; then, indicate the incorrect: *based on diff in EN of the two atoms
ionic: C-H>Li-H>H-F>Na-Cl>Rb-O :covalent
Which of the following properties is not determined by the electronic structure?
NoA
Which of the following characteristics is shared by tin, glass, cement, titanium, and carbon fiber ?
Their properties are influenced by their atomic structure, bonding, and atomic arrangement
Which of the following properties is not important for the material of an oven glove?
durability
For a magnetic soap holder with mildly alkaline soap, which would not be a design limiting factor
NoA
Most CDs are sold often crack and break easily. Which material property was likely neglected in their designs?
Fracture toughness
What kind of application require a material with high thermal conductivity?
cooking utensils, heat exchangers, heat sinks, thermal sensors
Which of the five modes of loading (tie, column, shell, beam or shaft) is dominant in a fizzy drink container?
Shell
Which of the five modes of loading (tie, column, shell, beam or shaft) is dominant in an ovhd electric cable?
tie
Which of the five modes of loading (tie, column, shell, beam or shaft) is dominant in a shoe sole?
column
Which of the five modes of loading (tie, column, shell, beam or shaft) is dominant in a wind turbine blade?
beam
Which of the following does not correspond to Tin?
Heat Insulator
T/F: Tmin is the temperature below which the material becomes brittle or otherwise unsafe to use
False
The diagram of the outdoor water tank, what aspects of the design might cause concern for corrosion? Indicate the incorrect
NoA; All are valid
Regarding the nernst equation: V = Vo + (0.0592/n)*log10(C); indicate the incorrect:
V is the corrected potential bc a concentration of 1M was not used
What material is most suitable for a cathode (most positive)?
Ag
What material is most suitable for an anode (most negative)?
Mg
Indicate the incorret for spontaneous rxns?
NoA
Indicate the incorrect statement:
Anions are equally attracted to the anode and cathode
Which of the following corresponds to a reduction reaction?
Some ions from the electrolyte electroplate into the metal
Indicate the correct regarding satd hydrocarbons
In saturated hydrocarbons each carbon is singly bonded to 4 other atoms
Indicate the incorrect
Unsaturated hydrocarbons cannot form new bonds
Indicate the incorrect
Bullet proof vests cannot be made of polyethylene
Indicate the incorrect regarding polymers
Polymer properties are not affected by the length of the polymer chains
Indicate the incorrect regarding polymers
MW affects polymers elastic modulus but not its strength
The description “is one in which the bonding along the ploymer chains is covalent and strong but the bonding between them is Van der Waals or hydrogen bonding and is weak. This causes the polymer to melt when heated, allowing thermal molding.” belongs to:
a thermoplastic
“it is a slightly cross-linked polymer which, at room temperature, is above its glass-transition temperature” belongs to
an elastomer
What is a specific heat
the energy to heat 1 Kg of material by 1 K
Regarding the specific heat per unit volume, and the specific heat per unit mass for solids, indicate the incorrect
Cv = (Cp)vol*p
Units for Cp
J/(kg*K)
If you wnated to select a material for a compact heat storing device, what would you use as a criterion of choice?
For a minimum volume the one with the highest (Cp)vol value.
The rate of diffusion of oxygen from the atmosphere into an oxide film is strongly dependent on the temperature and concentration of oxygen in the atmosphere. Indicate the incorrect
NoA
Diffusion is more rapid in the polycrystalline silver with a small grain size than in course-grained silver. indicate the incorrect
NoA
Carbon diffuses more rapidly than chromium through iron. Indicate the incorrect
The diffusion in iron is faster than the one of carbon in iron
Carbon diffuses more rapidly than chromium through iron. Indicate the incorrect
C atoms will not diffuse relatively rapidly
Regarding PCM indicate the incorrect
The latent heat is the heat adsorbed or released during a phase change,
Which of the following is incorrect wrt to heat capacity
NoA
Indicate the incorrect
whether we get a good cp or good cp vol, it is not required to get a minimum service temp
units for cp
kWh/(K*kg)
Conductor
Partly filled outer band, allowing electrons to move easily
Insulator
Have a filled outer band that is separated from the nearest unfilled band by a band gap
FIELDS CREATED BY NEIGHBORING ELECTRONS IN A CRYSTAL CAUSE…
ENERGY LEVELS OF INDIVIDUAL ATOMS TO SPLIT INTO BANDS OF CLOSELY SPACED LEVELS