Chem 2443 Super Assesment 2
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
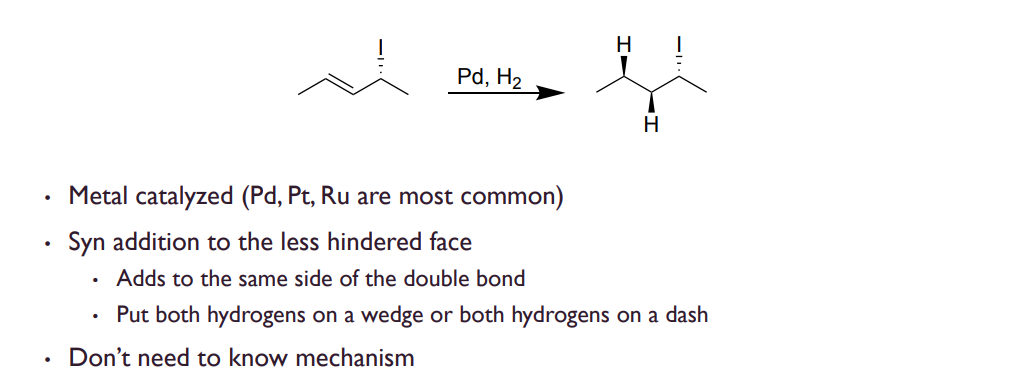
Hydrogenation
Catalyzed by palladium or platinum, adds syn addition from the LESS hindered side.
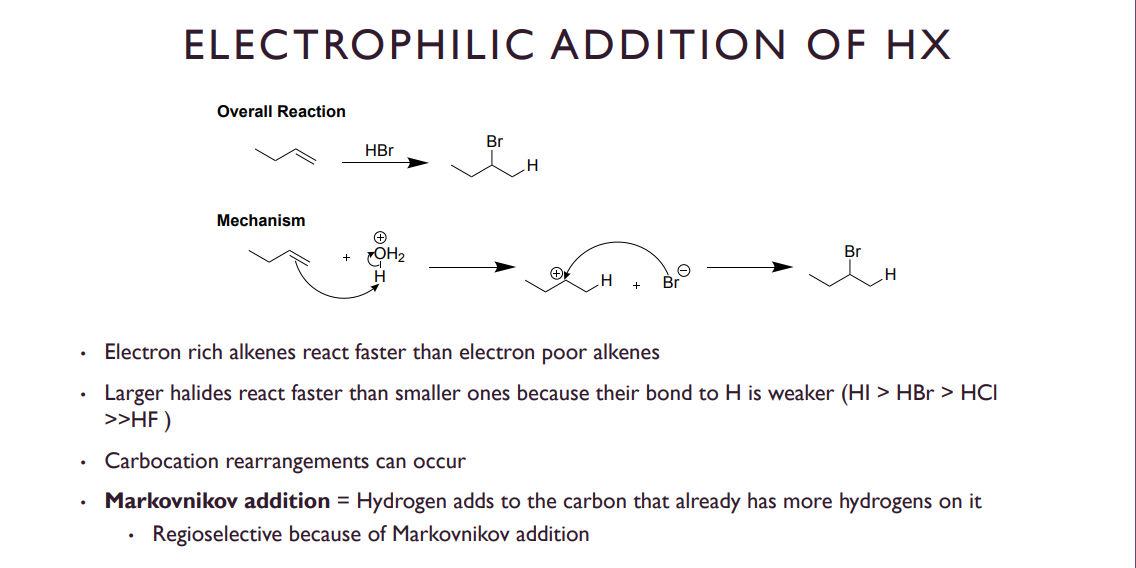
Electrophilic Addition Of HX
So, what we mean by more electron rich is essentially MORE substituted, making it more reactive. Stronger acids like HI will release their proton easier, and since this creates the carbocation it is our slowest, and RATE determining step.
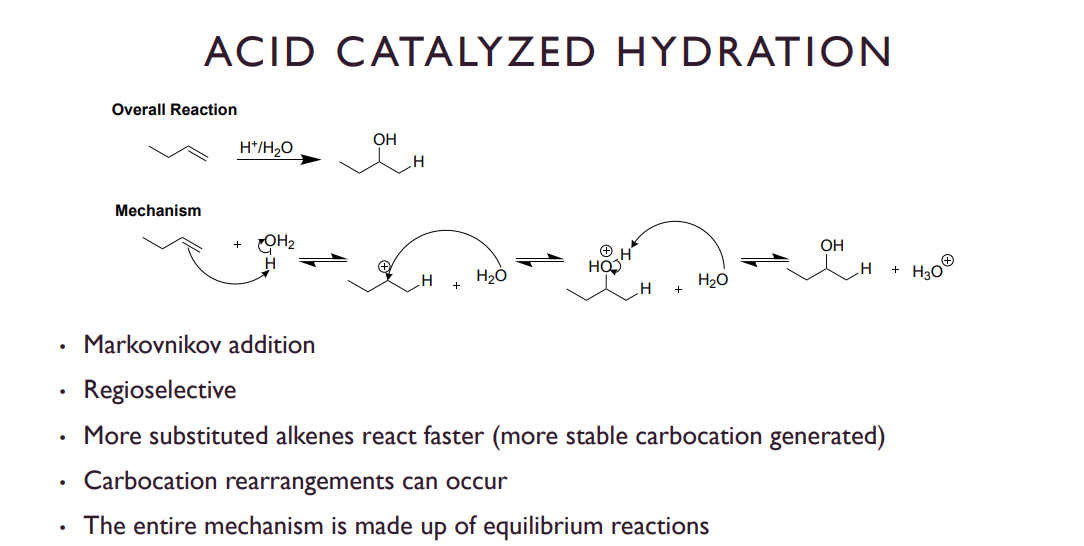
Acid-catalyzed hydration
Step 1)Protonation by acid, slow step
Step 2) Water acts as a nucleophile, creating an oxonium ion
Step 3) Deprotonation of the oxonium, leaving us with an OH group and a hydrogen
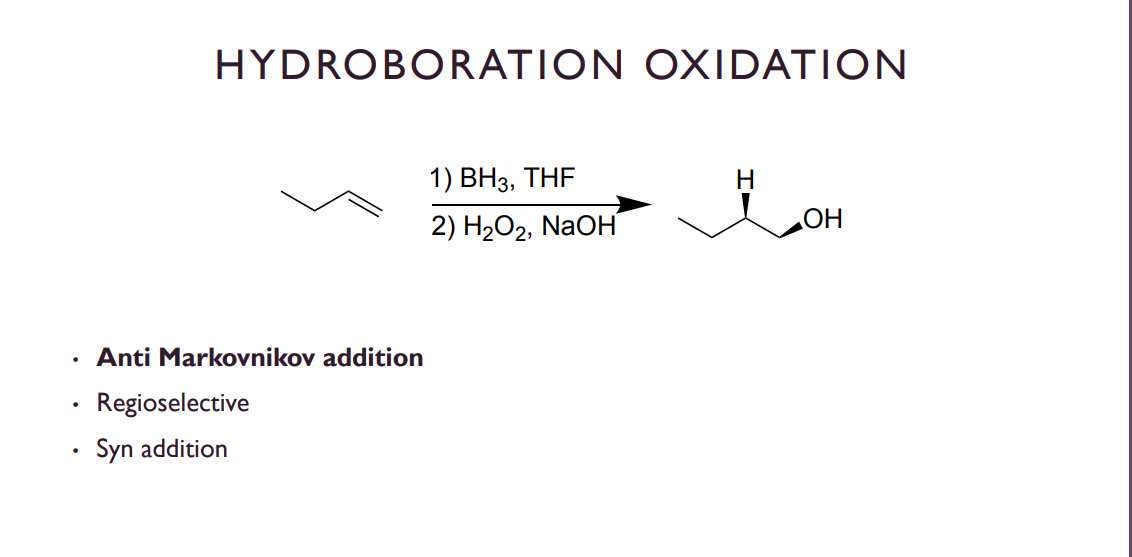
Hydroboration Oxidation
So in this reaction we have 2 major steps, the adding of a BH3 group which will re arrange into a BH2 which is then oxidized in step to and replaced by an OH group (aswell as the H that Leaves BH3). Because of this process it ends up being syn edition and ANTI markovnikov.
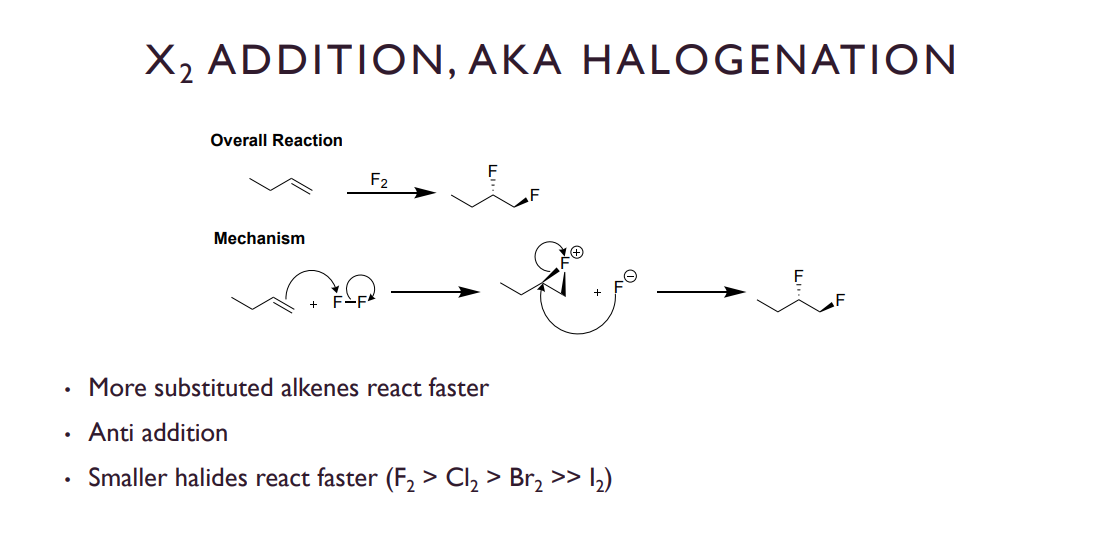
X2 ADDITION, AKA HALOGENATION
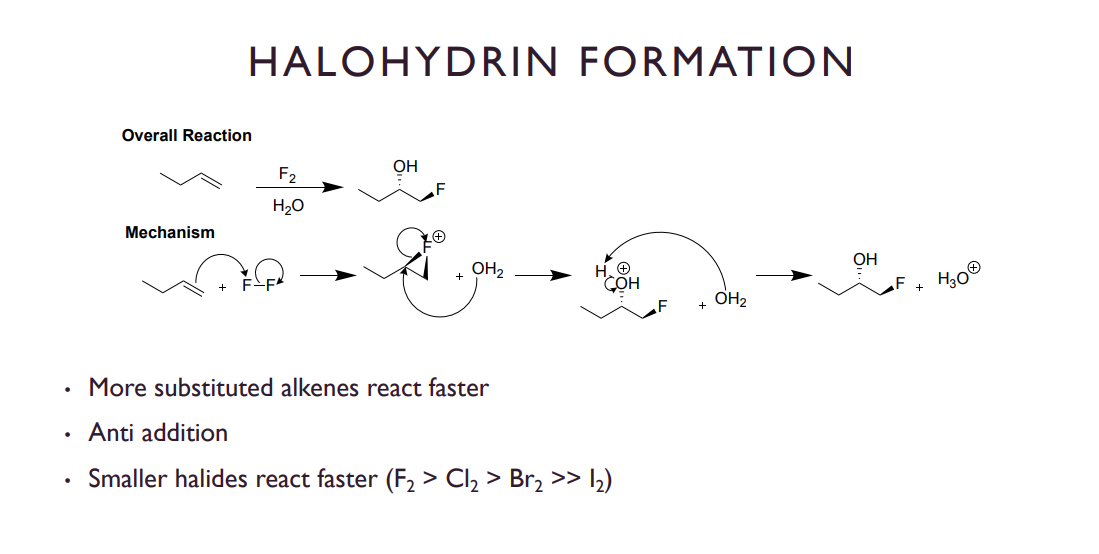
halohydrin formation
same as X2 addition except in an aqueous environment, so H2O is added instead of a second X and its deprotonated to form an alcohol group.
IMPORTANT NOTE: In this case it is REGIO SELECTIVE and the halogen will prefer the less substituted carbon while the OH will land on the more substituted carbon.
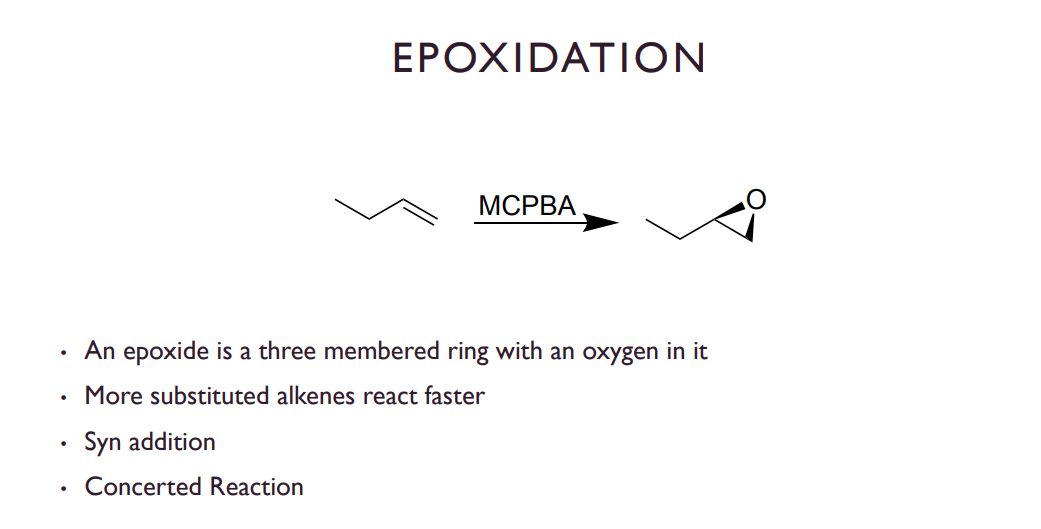
EPOXIDATION
Syn edition because other substituents dont move rly. 1 step concerted reaction
MCBPA or Peroxyacetic acid to donate an oxygen acting as an electrophile.
Ozonolysis

When would a carbocation rearrangement be a possibility?
Acid-catalyzed dehydration
Hydrogen Halide addition (hydrohalogenation)
Sn1 and E1 mechanisms.
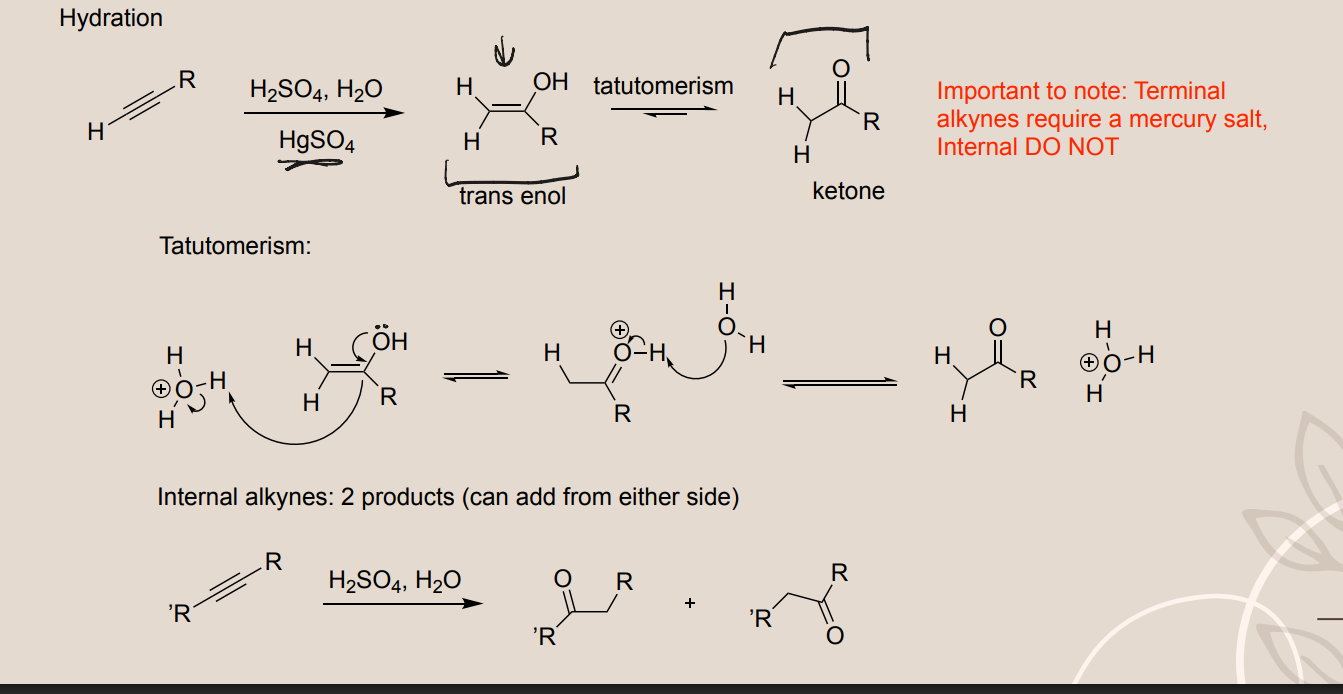
Alkyne Hydration
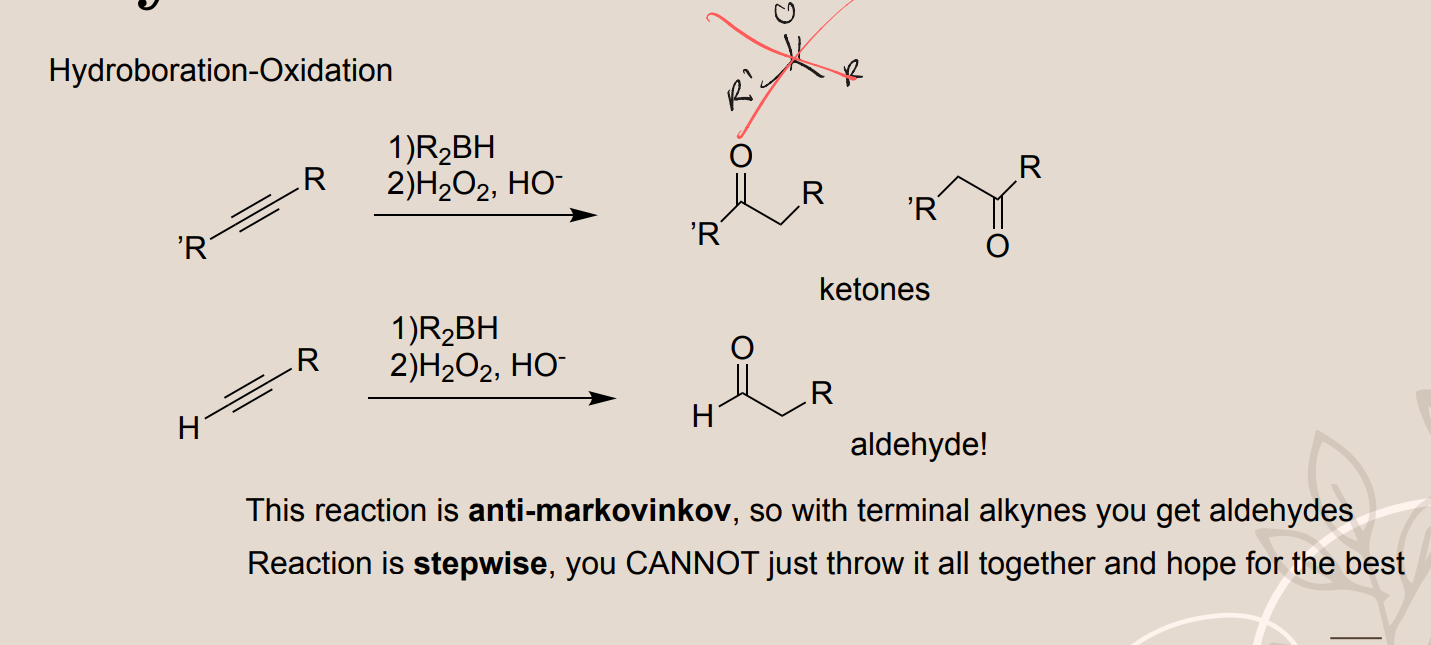
Alkyne Hydroboration Oxidation
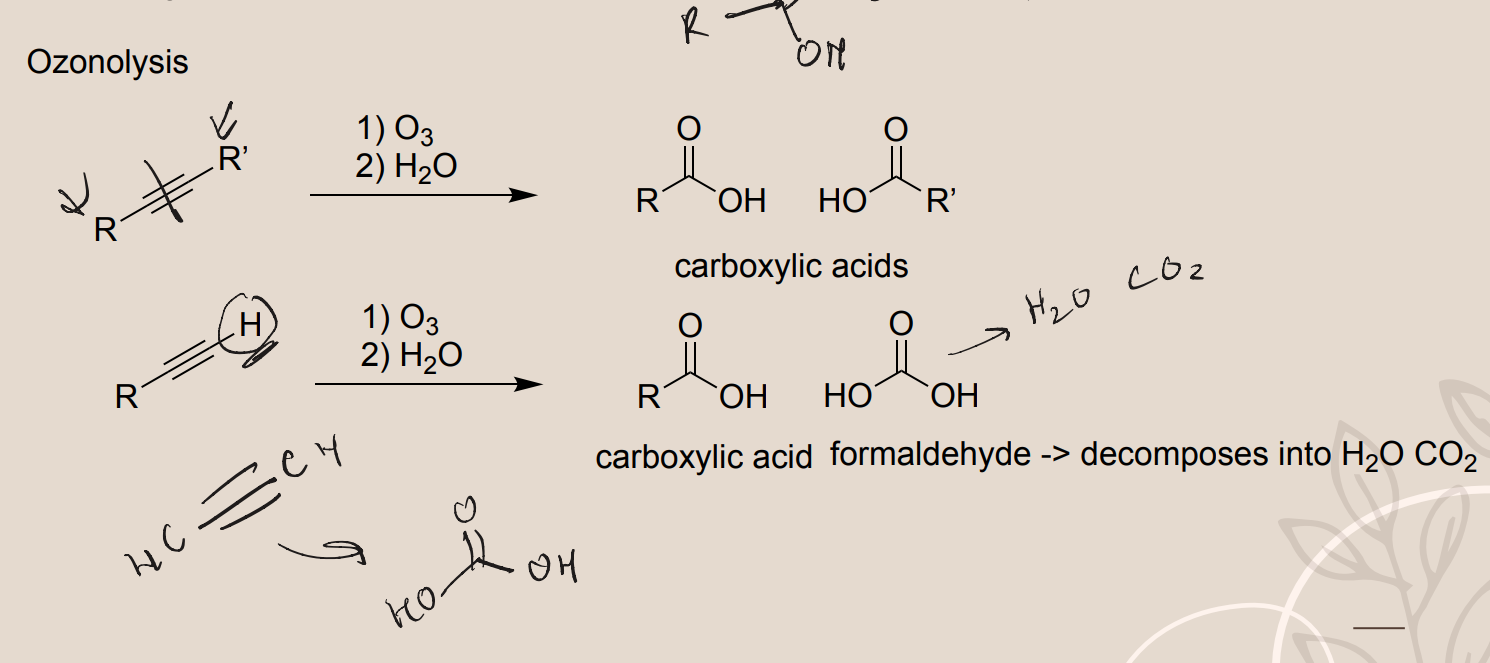
Ozonolysis of Alkynes
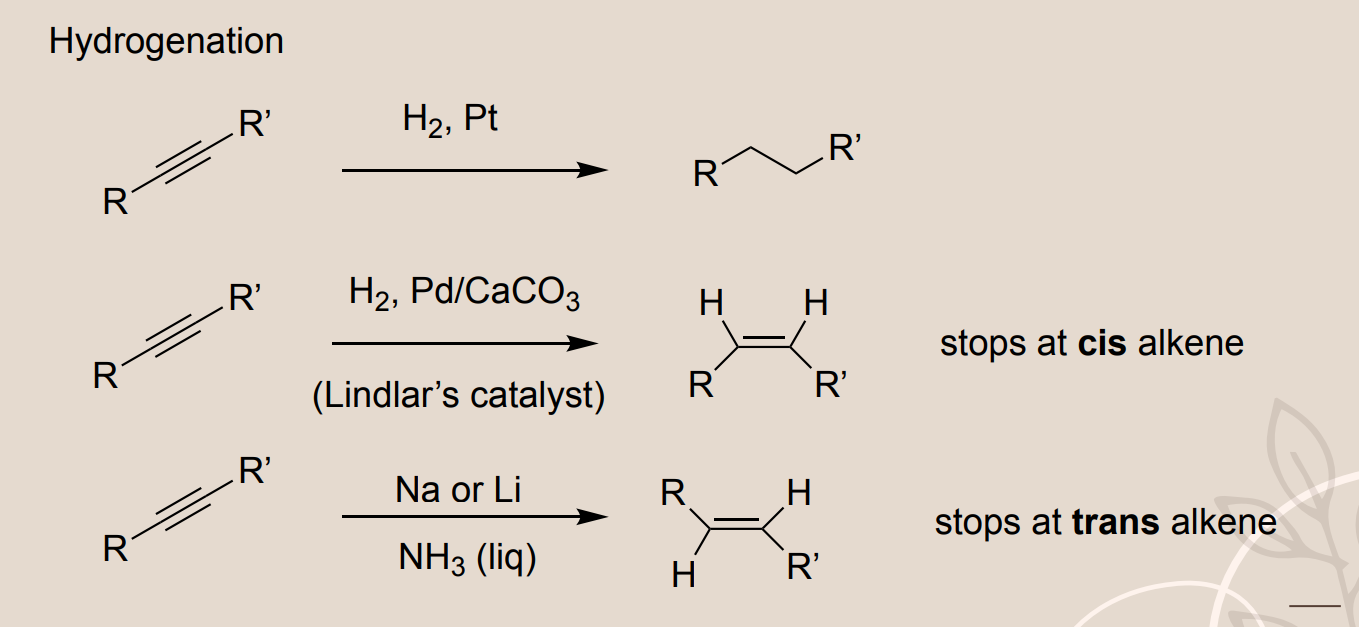
Hydrogenation of Alkynes
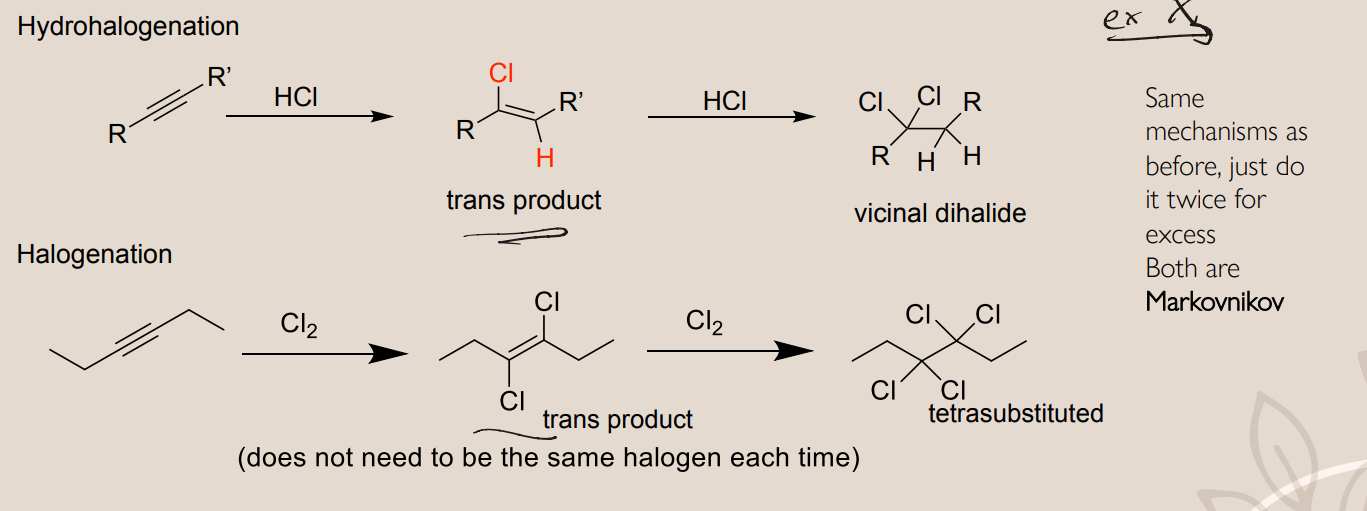
Alkyne Hydrohalogenation
Alkyne Preparation
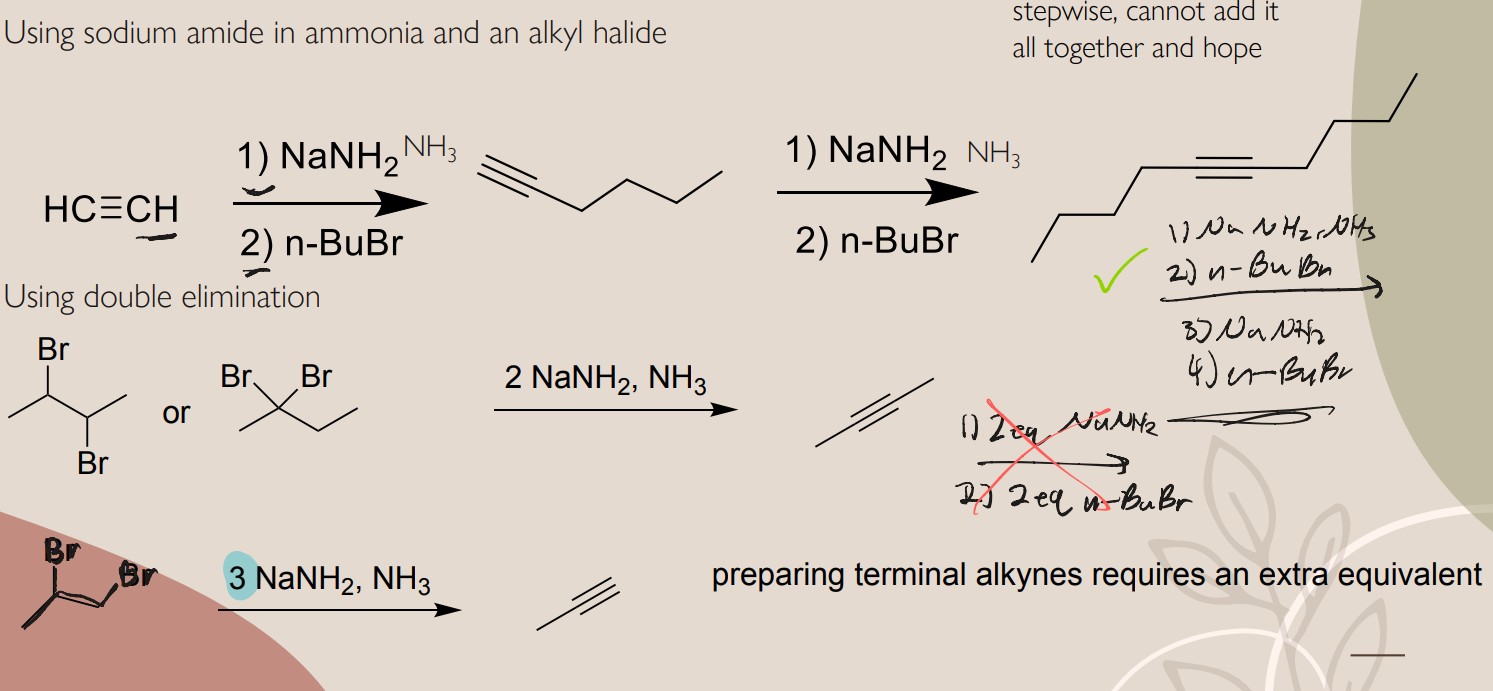
EAS halogenation.
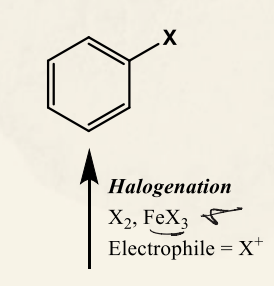
EAS nitration
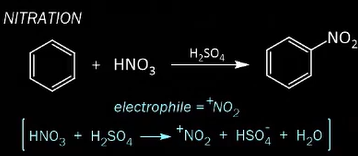
EAS Alkylation
Br uses FeBr3
If two substituents are ____ to each other, they are ______.
Ortho Right next to each other
Meta Separated by 1 carbon
Para Separated by 2 carbons
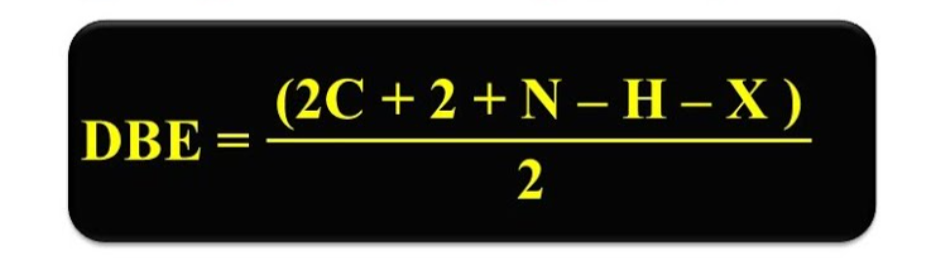
dbe formula
Primary Secondary Tertiary base and nucleophile strength.
