substitution and elimination reactions - orgo 1
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
3 polar protic solvents
water, ethanol, isopropanol
polar aprotic solvents
acetone, acetonitril, DMSO, DMF
strong nucleophiles (8)
I-, SH-,CN-, OH-, (CH3CH2)2NH, CH3O-, (CH3CH2)3P
what are the four things that make a nucelophile strong
-q
larger + very polarizable
not very electronegative
does not have bulky (alkyl) gRs on it (blocks it ability to get close to E+)
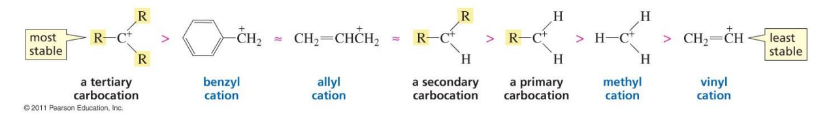
rank the stability of charged intermediates (carbocations)
mehtyl cation
primary cation
benzyl cation
allyl cation
tertiary cation
secondary cation
vinyl cation
weak nucleophiles
F- (very dense and not polarizable, very EN), H2O, (CH3)2O
moderate nucelophiles
Br-, NH3, (CH3)2S, Cl-, (CH3)COO-
good leaving groups
halogens, sulfate, sulfonate (e.g mesylate OMs, Tosylate Ots), water, amines (R3N), sulfides (R2S) or anything that is stable (CB of a WA)
strong bases
h-, nh2-
weak bases
dbu, dbn
strong nu/b
ho-, ch3o-, ch3ch2o-, tert-butoxide