Chapter 1 test chem
1/262
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
263 Terms
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
Discovered the electron
Thomson
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
Uncertainty principle; a particle’s position, energy and time can never be precisely known
Heisenberg
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
Created a model of the atom with electrons moving around the nucleus in fixed orbits
Bohr
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
Discovered the neutron
Chadwick
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
Did the gold foil experiment
Rutherford
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
hypothesized that the atom was a tiny hard sphere
John Dalton
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
believed that the universe was made of tiny “uncuttable” particles
Democritus
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
believed that atoms of a given element are identical
John Dalton
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
Greek philosopher
democritus
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
created the atomic theory
John Dalton
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
worked with the quantum theory
Heisenberg and Bohr
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
Used the cathode ray tube in his discovery
Thomson
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
Found the missing mass of the atom’s nucleus
Chadwick
Match the scientist:
John Dalton, Werner Heisenberg, J.J Thomson, Ernest Rutherford, Democritus, Niels Bohr, James Chadwick
Created the plum pudding model of the atom
Thomson
Who is responsible for this:
Stated that in a chemical reaction, atoms are combined, separated or rearranged
John Dalton
The statement "In a chemical reaction, atoms are combined, separated, or rearranged" was made by John Dalton.
This idea is part of Dalton's Atomic Theory, formulated in the early 1800s. His theory laid the groundwork for modern chemistry and included the concept that atoms are indivisible in chemical processes and simply rearranged during reactions, not created or destroyed.
Who is responsible for this:
Discovered the nucleus of the atom
James Chadwick
The neutron was discovered by James Chadwick in 1932.
He conducted experiments that showed the existence of a neutral particle in the nucleus of the atom, which helped explain the missing mass that couldn't be accounted for by protons alone. This discovery was crucial to understanding atomic structure and eventually led to the development of nuclear energy and atomic weapons.
Who is responsible for this:
Stated that atoms of a particular atom are all alike
John Dalton
The statement "Atoms of a particular element are all alike" was made by John Dalton.
This was part of Dalton's Atomic Theory (early 1800s), which proposed that:
All matter is made of atoms.
Atoms of a given element are identical in mass and properties.
Atoms of different elements differ in mass and properties.
Atoms cannot be subdivided, created, or destroyed in chemical reactions.
Atoms combine in simple whole-number ratios to form compounds.
So, the correct answer is: John Dalton.
Who is responsible for this:
First discovered there are particles smaller than an atom
thomson
The scientist who first discovered that there are particles smaller than an atom was J.J. Thomson.
In 1897, he discovered the electron through his experiments with the cathode ray tube. This showed that atoms are not indivisible, as previously thought, but contain smaller subatomic particles — specifically, negatively charged electrons.
So, the correct answer is: J.J. Thomson.
Who is responsible for this:
Stated that atoms of different elements combine in small whole number ratios
The statement "Atoms of different elements combine in small whole number ratios" was made by John Dalton.
This is part of Dalton's Atomic Theory, specifically addressing how atoms form compounds:
Atoms of different elements combine in simple whole-number ratios to form chemical compounds.
So, the correct answer is: John Dalton.
Who is responsible for this:
Discovered that atoms of each element contain a unique + charge in nuclei
The scientist who discovered that atoms of each element contain a unique positive charge in their nuclei was Ernest Rutherford.
In his gold foil experiment (1909), Rutherford:
Discovered the nucleus of the atom.
Showed that the positive charge (protons) is concentrated in a small central nucleus.
Found that each element has a unique number of protons in its nucleus, later called the atomic number.
Although Henry Moseley later refined this idea by measuring the atomic number through X-ray spectroscopy, Rutherford was the first to discover that the positive charge is in the nucleus and varies by element.
So, the correct answer is: Ernest Rutherford.
Whos responsible for this:
Discovered the charge to mass ratio of the electron
The scientist who discovered the charge-to-mass ratio of the electron was J.J. Thomson.
In 1897, using a cathode ray tube, Thomson measured how the rays (electrons) were deflected by electric and magnetic fields. From this, he was able to calculate the charge-to-mass ratio (e/m) of the electron — even though he didn’t yet know the actual charge or mass individually.
So, the correct answer is: J.J. Thomson.
Whos responsible for this:
Stated that atoms cannot be subdivided, created or destroyed
The statement "Atoms cannot be subdivided, created, or destroyed" was made by John Dalton.
This idea is part of Dalton’s Atomic Theory (early 1800s), which proposed that:
All matter is made of atoms.
Atoms are indivisible and indestructible in chemical processes.
Chemical reactions involve the rearrangement of atoms, but the atoms themselves are not changed.
So, the correct answer is: John Dalton.
Whos responsible for this:
Discovered the neutral particle in the nucleus
The scientist who discovered the neutral particle in the nucleus—the neutron—was James Chadwick.
He made this discovery in 1932, proving that the nucleus contains not just positively charged protons but also neutral particles, which explained the missing mass in the nucleus.
So, the correct answer is: James Chadwick.
Whos responsible for this:
Stated that atoms of one element are different from atoms of another element
The statement "Atoms of one element are different from atoms of another element" was made by John Dalton.
This is a key part of Dalton’s Atomic Theory, which proposed that atoms of different elements have different masses and properties, distinguishing one element from another.
So, the correct answer is: John Dalton.
Who responsible for this:
Discovered the charge on an electron
The charge of the electron was discovered by Robert Millikan through his famous oil drop experiment in 1909.
Millikan measured the exact value of the electron's charge, which allowed scientists to understand the fundamental charge of this subatomic particle.
So, the correct answer is: Robert Millikan.
Who responsible for this:
First stated that all matter was made of indivisible atoms
The first person to state that all matter is made of indivisible atoms was the ancient Greek philosopher Democritus.
Around 400 BCE, Democritus proposed that matter is composed of tiny, indivisible particles called "atomos," meaning uncuttable or indivisible.
So, the correct answer is: Democritus.
Atomic theory matching:
1. This device gave scientists their first image of individual atoms
2. He confirmed the existence of the neutron
3. He developed the beginnings of the modern atomic theory in the 1800s
4. He developed an elaborate experiment that showed that the majority of an atom is empty
5. He first suggested the presence of atoms and named them atomos (“indivisible”)
6. He used a cathode ray tube to discover electrons
A. Democritus B. John Dalton C. J.J Thomson D. James Chadwick E. Ernest Rutherford F. Scanning Tunneling Microscope (STM)
Here’s the matching for the atomic theory statements with the options given:
This device gave scientists their first image of individual atoms
→ F. Scanning Tunneling Microscope (STM)He confirmed the existence of the neutron
→ D. James ChadwickHe developed the beginnings of the modern atomic theory in the 1800s
→ B. John DaltonHe developed an elaborate experiment that showed that the majority of an atom is empty
→ E. Ernest RutherfordHe first suggested the presence of atoms and named them atomos (“indivisible”)
→ A. DemocritusHe used a cathode ray tube to discover electrons
→ C. J.J Thomson
What does quantum mean? How does this relate to electrons?

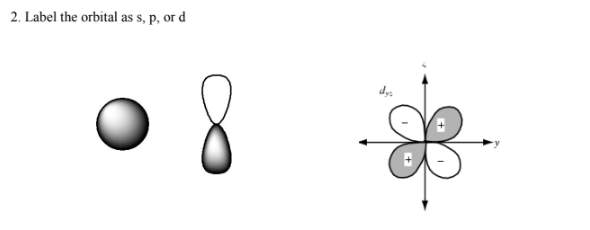
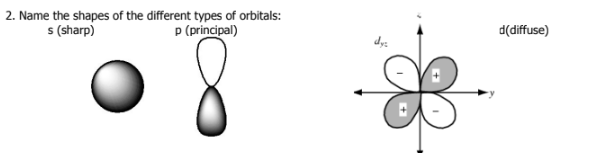
What are the four quantum numbers called?
The principle quantum number (n)
The secondary quantum number (l)
The magnetic quantum number (ml)
The magnetic spin quantum number (ms)
Which quantum number determine s the type of orbital shape?
Secondary quantum number (l)
What does the principle quantum number (n) describe
energy of electron
size of shell
describes orbital
What does the secondary quantum number (l) describe
shape of orbital →describes orbital
What does the magnetic quantum number (ml) describe
orientation of orbital → describes orbital
What does the magnetic spin quantum number (ms) describe
“spin” of electron →describes electron
For n = 3, what are the possible values of l?

For l = 2, what are the possible values of ml

Give the numerical values of n and l corresponding the each of the following designations:
a) 3p
b) 2s
c) 4d

Give the values for n, l and ml for each orbital in the 3p subshell

Which of the following represent impossible combinations of n and l
a) 1p
b) 4s
c) 5d
d) 2d
a) and d) don’t exist on p table
a) List the four quantum numbers for each electron in an N atom

b) List the four quantum numbers for the valence electrons in Ca

Give the values of all the quantum numbers that can be associated with an electron in:
a) a 3p orbital
b) a 5d orbital
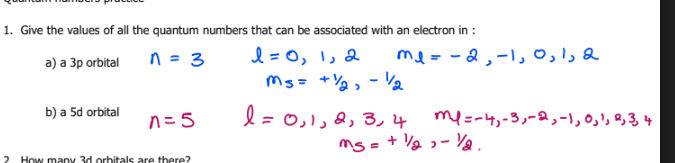
How many 3d orbitals are there?
5
How many 4p orbitals are there
3
What is the total number of orbitals associated with the principle quantum number
a) n =2
b) n = 3
c) n = 4
chatgpt
just square each number
Draw the orbital diagram and write the electron configuration:
1H

Draw the orbital diagram and write the electron configuration:
2He

Draw the orbital diagram and write the electron configuration:
3Li

Draw the orbital diagram and write the electron configuration:
4Be

Draw the orbital diagram and write the electron configuration:
5B
Draw the orbital diagram and write the electron configuration:
6C

Draw the orbital diagram and write the electron configuration:
7N

Draw the orbital diagram and write the electron configuration:
8O

Draw the orbital diagram and write the electron configuration:
9F
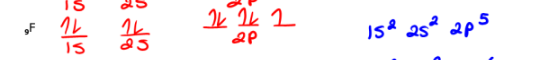
Draw the orbital diagram and write the electron configuration:
10Fe

Draw the orbital diagram and write the electron configuration:
11Na

Write the electron configuration of each of the following using the noble gas method
53I
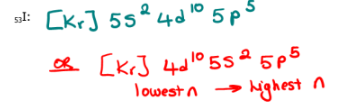
Write the electron configuration of each of the following using the noble gas method
57La
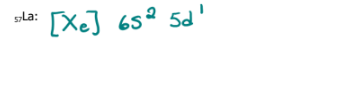
Write the electron configuration of each of the following using the noble gas method
60Nd
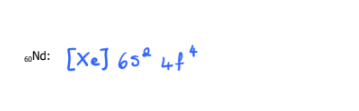
Write the electron configuration of each of the following using the noble gas method
76Os
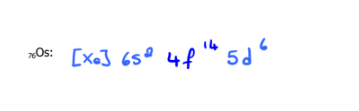
Write the electron configuration of each of the following using the noble gas method
79Au

Write the electron configuration of each of the following using the noble gas method
82Pb2+
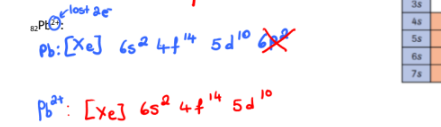
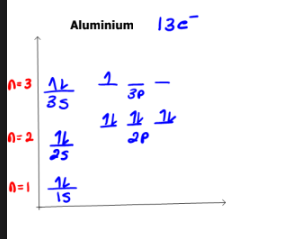
Draw the orbital diagram for aluminum
Draw the orbital diagram for fluorine
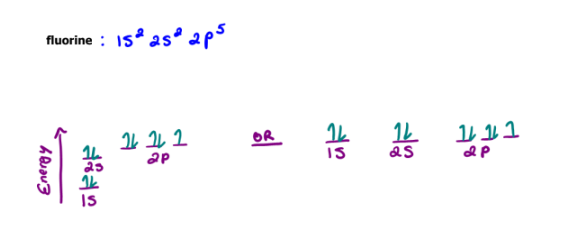
Do the electron configuration and draw the orbital diagram
Ar
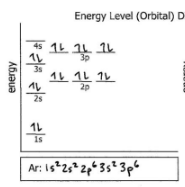
Do the electron configuration and draw the orbital diagram
N
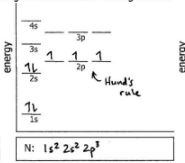
Do the electron configuration and draw the orbital diagram
P
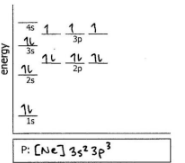
Do the electron configuration and draw the orbital diagram
Na
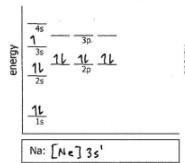
Do the electron configuration and draw the orbital diagram
Ca
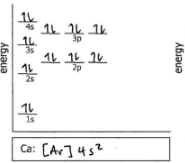
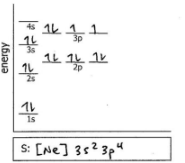
Do the electron configuration and draw the orbital diagram
S
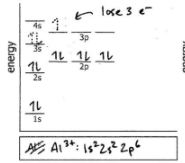
Do the electron configuration and draw the orbital diagram
Al3+
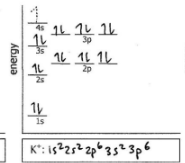
Do the electron configuration and draw the orbital diagram
K+
Do the electron configuration and draw the orbital diagram
Cl-

Do the electron configuration and draw the orbital diagram
S2-
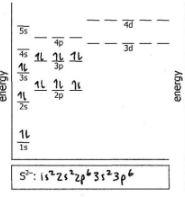
Do the electron configuration and draw the orbital diagram
Zn2+
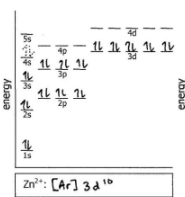
Do the electron configuration and draw the orbital diagram
Ag+
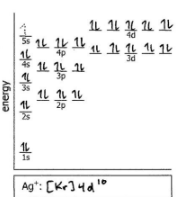
Do the electron configuration and draw the orbital diagram
As
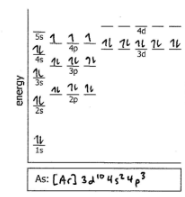
Do the electron configuration and draw the orbital diagram
As3+
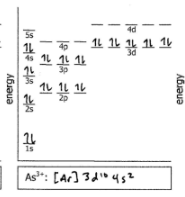
Do the electron configuration and draw the orbital diagram
As5+
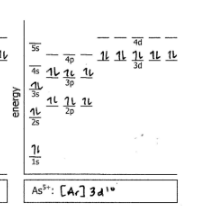
Do the electron configuration and draw the orbital diagram
Fe
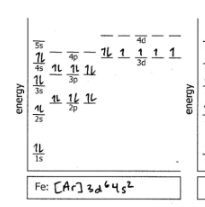
Do the electron configuration and draw the orbital diagram
Fe2+
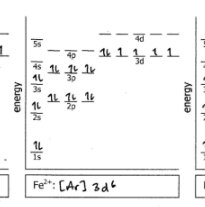
Do the electron configuration and draw the orbital diagram
Fe3+
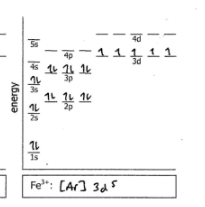
Do the electron configuration and draw the orbital diagram
Sn4+
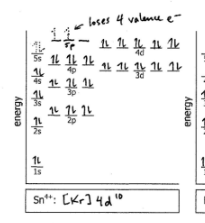
Do the electron configuration and draw the orbital diagram
Mn2+
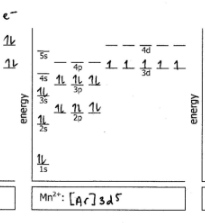
Do the electron configuration and draw the orbital diagram
Cr6+
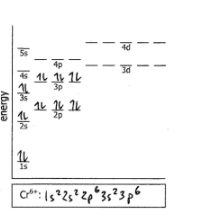
True or False
The volume of an atom is essentially that volume occupied by the electrons
True.
The volume of an atom is essentially the volume occupied by its electrons.
Explanation:
Atoms are mostly empty space.
The nucleus (protons and neutrons) occupies a very small central region.
The electrons are spread out in a "cloud" around the nucleus, and this cloud defines the effective size or volume of the atom.
Even though electrons are tiny and have negligible mass compared to the nucleus, the region where they are likely to be found (according to quantum mechanics) determines the atom’s spatial extent.
So, the volume of an atom refers to the space where its electrons are likely to be found, not the physical volume of its subatomic particles.
True or False
The greater the difference in energy between two levels, the longer the wavelength of the light emitted when an electron moves between them
False.
Correct Explanation:
The greater the energy difference between two levels, the shorter the wavelength of the light emitted.
This follows from the equation:
E = hc/λ
Where:
E = energy difference between levels
h = Planck's constant
c = speed of light
λ = wavelength
So, if energy E increases, then wavelength λ must decrease (they are inversely proportional).
Therefore:
Large energy difference → short wavelength (e.g. ultraviolet)
Small energy difference → long wavelength (e.g. infrared)
✅ Final Answer: False
True or False
The atomic number is always equal to the number of electrons in a particular atom
False.
Explanation:
The atomic number of an element is equal to the number of protons in its nucleus.
In a neutral atom, the number of electrons equals the number of protons, so the atomic number does equal the number of electrons in that case.
However, if the atom is ionized (it gains or loses electrons), then the number of electrons is not equal to the atomic number.
Examples:
Neutral sodium atom (Na):
Atomic number = 11 → 11 protons and 11 electrons ✅
Sodium ion (Na⁺):
Still has 11 protons → atomic number = 11
But now has 10 electrons ❌
✅ Final Answer: False — because the atomic number equals the number of electrons only in neutral atoms, not always.
True or False
The radius of a negatively charged monatomic ion is larger than the radius of the parent neutral atom
True.
Explanation:
When a neutral atom gains one or more electrons to become a negatively charged ion (anion):
Electron-electron repulsion increases, because more electrons are present in the same electron cloud.
The effective nuclear charge per electron decreases, since the number of protons (positive charge) stays the same, but more electrons are present.
As a result, the electron cloud expands, and the ion becomes larger than its neutral parent atom.
Example:
Cl (neutral atom): has 17 protons and 17 electrons.
Cl⁻ (anion): has 17 protons and 18 electrons → increased repulsion causes a larger radius.
✅ Final Answer: True — the radius of a negatively charged monatomic ion is larger than that of its neutral atom.
True or False
The energy associated with an electron in a given atom is almost fully described by specifying its value of the quantum number n
In more detail:
In the hydrogen atom (which has only one electron), the energy levels depend only on the principal quantum number nnn:
En = -3.6eV / n2
✅ So for hydrogen, n does describe the energy completely.
In multi-electron atoms, energy also depends on the azimuthal quantum number ℓ\ellℓ, which determines the subshell (s, p, d, f):
Orbitals with the same nnn but different ℓ\ellℓ values (like 2s and 2p) have different energies.
This is due to electron-electron interactions and shielding effects.
Conclusion:
For hydrogen: nnn is enough → True in this limited case.
For most atoms: nnn is not sufficient → must include ℓ\ellℓ and possibly other quantum numbers.
✅ Final Answer: False — because nnn alone does not fully describe the energy in most atoms.
True or False
An orbital diagram is a geometrical representation of the shape of an orbital
Explanation:
An orbital diagram is not a geometrical representation of an orbital's shape. Instead:
An orbital diagram is a graphical way to show electron configurations.
It uses boxes or lines to represent orbitals and arrows to represent electrons (with their spins
In contrast, a geometrical representation of an orbital's shape refers to the actual spatial distribution of where an electron is likely to be found — like:
s orbitals: spherical
p orbitals: dumbbell-shaped
d orbitals: cloverleaf-shaped
These are often shown as 3D plots of probability densities or boundary surfaces.
✅ Final Answer: False — an orbital diagram shows electron configurations, not orbital shapes.
True or False
The energy associated with electromagnetic radiation decreases in order x-ray > ultraviolet > visible > infared
True.
Explanation:
The energy of electromagnetic radiation is inversely proportional to its wavelength, according to the equation:
E = hc / λ
Where:
E = energy
h = Planck's constant
c = speed of light
λ = wavelength
So, shorter wavelength = higher energy.
Electromagnetic spectrum (from high to low energy):
X-rays → shortest wavelength → highest energy
Ultraviolet (UV)
Visible light
Infrared (IR) → longest wavelength among these → lowest energy
✅ Final Answer: True — energy decreases in the order:
x-ray > ultraviolet > visible > infrared.
True or False
When an atom absorbs a photon of energy E, the atom undergoes an increase in energy equal to or less than E
False.
Explanation:
When an atom absorbs a photon of energy EEE, the atom’s energy increases by exactly EEE — not less than EEE.
This is because:
Photons are quantized packets of energy.
An atom can only absorb a photon if the photon's energy exactly matches the energy difference between two allowed energy levels.
If the energy doesn’t match an allowed transition, the photon is not absorbed.
ΔEatom = ΔEphoton
✅ Final Answer: False — the atom’s energy increases by exactly E, not less than E.
True or False
Ground state describes an atom with all its electrons in the lowest possible energy levels (and sublevels)
Explanation:
The ground state of an atom refers to the condition where all its electrons are in the lowest possible energy levels and sublevels available, consistent with the rules of:
The Aufbau principle (fill lowest energy orbitals first),
The Pauli exclusion principle (no two electrons can have the same set of quantum numbers), and
Hund's rule (electrons occupy degenerate orbitals singly before pairing).
If an atom is not in the ground state (e.g., one or more electrons are in higher energy orbitals than necessary), it is said to be in an excited state.
✅ Final Answer: True — the ground state means all electrons are in the lowest energy levels and sublevels possible.
True or False
Gravitational attraction makes a negligibly small contribution to the force holding an electron in an atom
Explanation:
The force that holds an electron in an atom is primarily the electrostatic (Coulomb) attraction between the negatively charged electron and the positively charged nucleus.
While there is technically a gravitational force between the electron and the nucleus, it is:
Extremely small compared to the electrostatic force.
Negligible in atomic-scale interactions.
This is because gravity is much weaker than the electromagnetic force by a factor of approximately 103610^{36}1036.
✅ Final Answer: True — gravitational attraction contributes a negligibly small amount to the force holding an electron in an atom.
Multiple choice
What kind of attractive force holds together the components of an individual atom
a) gravitational
b) magnetic
c) electrical
d) the chemical bond
c) electrical
Explanation:
The components of an atom (protons, neutrons, electrons) are held together primarily by electrical (electrostatic) forces:
The electrons are attracted to the positively charged nucleus due to electrostatic attraction.
Gravitational forces are negligible at atomic scales.
Magnetic forces are not the primary force holding an atom together.
Chemical bonds hold atoms together in molecules, not the components within an atom.
Multiple choice
Experimental support for the arrangement of electrons in distinct energy levels is based primarily upon
a) the law of constant composition
b) the law of conservation of energy
c) continuous spectra
d) spectra from electrical discharge through gases
d) spectra from electrical discharge through gases
Explanation:
When an electric current passes through a gas at low pressure, the gas emits light at specific discrete wavelengths—these are called line spectra or emission spectra.
These discrete lines correspond to electrons moving between distinct energy levels in the atoms.
This experimental evidence was crucial in supporting the idea that electrons exist in quantized energy levels, not a continuous range of energies.
a) Law of constant composition relates to chemical compounds having fixed ratios, not electron energy levels.
b) Law of conservation of energy is fundamental but not specific evidence for electron arrangement.
c) Continuous spectra come from sources like incandescent solids, not gases, and don’t show discrete energy levels.
Multiple choice
For an electron with a quantum number l = 2, the quantum number ml can have
a) only one value
b) any one of three values
c) any one of five values
d) an infinite number of values
The correct answer is:
c) any one of five values
Explanation:
The magnetic quantum number mlm_lml depends on the azimuthal quantum number lll.
For a given lll, mlm_lml can take integer values from −l-l−l to +l+l+l, including zero.
So, for l=2l = 2l=2, possible mlm_lml values are:
−2,−1,0,+1,+2,-2,
That’s a total of 5 possible values.
Multiple choice
The number of electrons which can be accommodated in an electronic sublevel with l = 2 is
a) 2
b) 6
c) 10
d) 14
c) 10
Explanation:
For l=2l = 2l=2, the sublevel corresponds to a d-sublevel.
The number of orbitals in a sublevel is given by the number of possible mlm_lml values:
ml=−l,...,0,...,+lm_l = -l, ..., 0, ..., +lml=−l,...,0,...,+l
For l=2l = 2l=2, there are 5 orbitals.
Each orbital can hold 2 electrons (with opposite spins).
So total electrons = 5×2=105 \times 2 = 105×2=10.