Thermal Physics
1/25
Earn XP
Description and Tags
Textbook chapter 14
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Sketch a diagram to illustrate the solid phase of matter and describe the arrangement, motion and forces between atoms/molecules in this phase.
In solid the particles (atoms or molecules) are regularly arranged and packed closely together.
There are strong electrostatic forces of attraction between the particles which hold them in fixed positions
The particles can vibrate, but they cannot move out of their positions in the structure.
Because the particles are closely packed together, a solid tends to occupy a smaller volume than the same mass of liquid or gas (there are exceptions, such as water). As such the density of a solid tends to be higher than a liquid and gas.
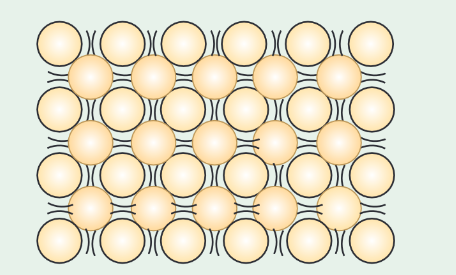
Sketch a diagram to illustrate the liquid phase of matter and describe the arrangement, motion and forces between atoms/molecules in this phase.
The particles in a liquid are close together but they can change positions and flow past each other.
A liquid therefore flows easily and has no fixed shape.
There are still forces of attraction between the particles, the particles have enough kinetic energy to not be held in fixed positions by these forces.
Because the particles in a liquid are much closer together than in a gas, a liquid occupies a much smaller volume than the same mass of gas. As such the density of a liquid tends to be much higher than a gas.
Because the particles in a liquid tend to be further apart than in a solid, a liquid occupies a larger volume than the same mass of solid. As such the density of a liquid tends to be lower than a solid.
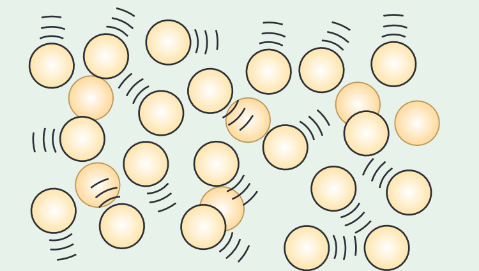
Sketch a diagram to illustrate the gas phase of matter and describe the arrangement, motion and forces between atoms/molecules in this phase.
The particles in a gas are much further apart and are free to move past each other as there are negligible electrostatic forces between them, unless they collide with each other or the container walls.
They move randomly with different speeds in different directions.
Because the particles are so far apart, a gas occupies a much larger volume than the same mass of liquid or solid. As such the density of a gas is much lower than a liquid or a solid.
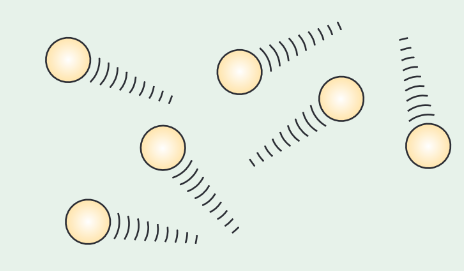
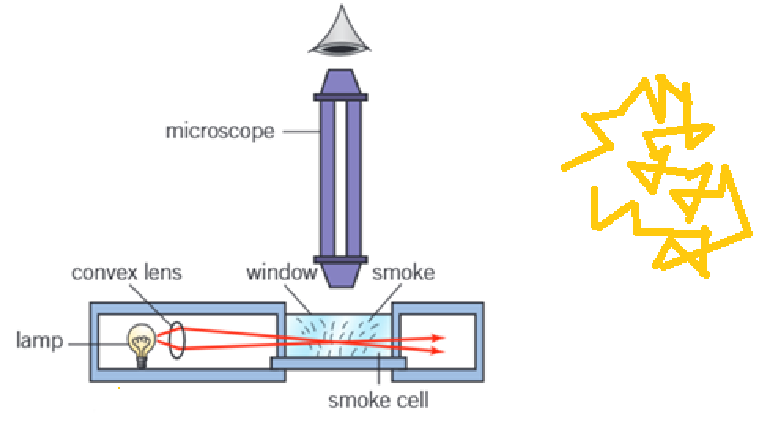
Describe the Brownian motion experiment and observations.
Robert Brown originally look at the motion of fine pollen grains on the surface of water through a microscope.
Brown observed the pollen grains moving randomly, similar to the yellow squigle shown in the image.
To observe brownian motion in the lab, a setup using a smoke cell can be used instead, as shown in the diagram.
The motion of the smoke particles will be similarly random.
What are the observations of the Brownian motion experiment?
The smoke / pollen particles are always moving…
… in random directions…
…in a jittery motion…
… following an erratic zigzag path
What deductions can be made from the observations of the Brownian motion experiment?
Air consists of a very large number of tiny particles: we know these to be molecules. These:
have a very small mass compared to the smoke particles.
are moving continuously therefore collisions are elastic
are moving in random directions
must have a range of speeds from zero to high speeds
How did Einstein explain Brownian motion and what conlusions did he draw?
Einstein explained Brownian motion in terms of collisions between the pollen grains and millions of tiny water molecules.
He explained that these collisions were elastic and resulted in a transfer of momentum from the water molecules to the pollen grains, causing the grains to move in haphazard ways.
This provided the first significant proof of the kinetic model - the idea that matter is made up of atoms and molecules and they have kinetic energy.
How is temperature different to thermal energy?
Temperature is a measure of the average kinatic energy of the particles in a substance, whereas thermal energy is a measure of the total kinetic energy of all the particles in a substance
This means temperature does not depend on the amount of substance you have, but thermal energy does
How is temperature linked to thermal energy?
Temperature affects thermal energy - the higher the temperature of a substance, the hinger the thermal energy.
However, thermal energy also depends on mass.
What is absolute zero?
The temperature at which substances have minimum internal energy. It is the lowest possible temperature.
How do you convert a temperature from the Celsius scale to the Kelvin / absolute temperature scale?
T(K) = θ(℃) + 273.16
What is thermal equilibrium?
A state in which there is no net flow of thermal energy between the objects involved, that is, objects in thermal equilibrium must be at the same temperature
What is the triple point of a substance?
For a given substance, the one specific temperature and pressure where the three phases of that substance can exist in thermal equilibrium.
What is the definition of internal energy?
The sum of the randomly distributed kinetic and potential energies of molecules within the substance.
Symbol: U, unit: joule (J).
What is latent heat?
The amount of energy per unit mass to change the phase of a substance.
What is the latent heat of fusion?
The energy required per unit mass to change a substance from a solid to a liquid at a constant temperature
What is the latent heat of vaporisation?
The energy required per unit mass to change a substance from a liquid to a gas at a constant temperature
Sketch a graph of how temperature varies with time as a substance changes phase from solid to liquid to gas. Label latent heat of fusion and vaporisation.
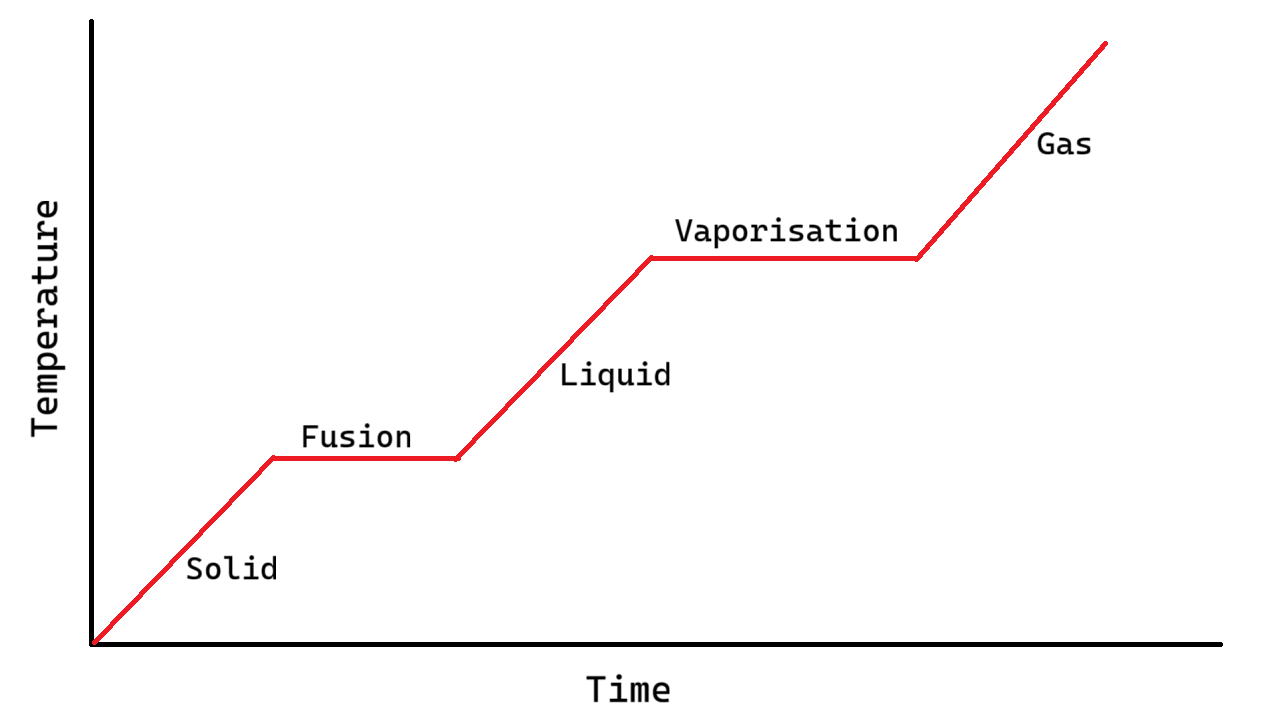
Describe the internal energy changes in a phase change
When a substance changes phase, the temperature does not change, nor does the kinetic energy of the atoms or molecules.
However, their electrostatic potential energy increases significantly as the electrical forces between the molecules change.
Only once the phase change is complete does the kinetic energy of the molecules increase further, and so the temperature rises rises again.
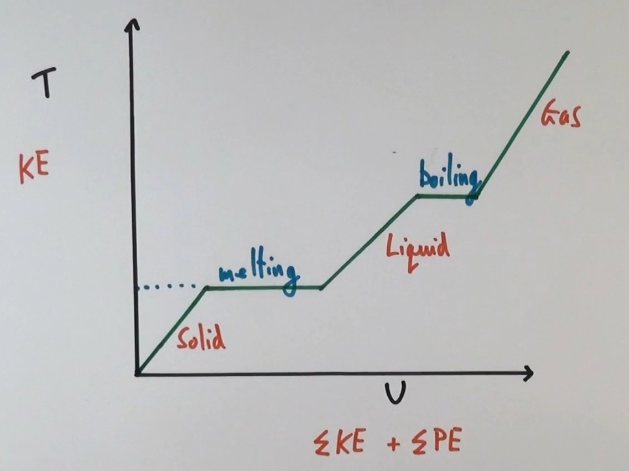
Describe the electrostatic forces and electrostatic potential energy in each phase of matter
Gas: The electrostatic potential energy is zero because there are negligible electrical forces between atoms or molecules.
Liquid: The electrostatic forces between atoms or molecules give the electrostatic potential energy a negative value. The negative simply means that energy must be supplied to break the atomic or molecular bonds.
Solid: The electrostatic forces between atoms or molecules are very large, so the electrostatic potential energy has a large negative value.
Why can’t zero internal energy be reached?
At absolute zero, the energy of a substance is a minimum. The kinetic energy of all the atoms or molecules is zero - they have stopped moving.
However, the internal energy is not zero because the substance still has electrostatic potential energy stored between the particles.
Even at 0K, you cannot reduce the potential energy of the substance to zero. As such the internal energy can’t be zero.
What is the specific heat capacity equation, inlcuding the meaning of each term and their units?
E = mcΔθ
E is the energy supplied to the substance in joules (J)
m is the mass of the substance in kilograms (kg)
Δθ is the change in temperature of the substance. This can be measured in K or ℃, since both give the same numerical value for change.
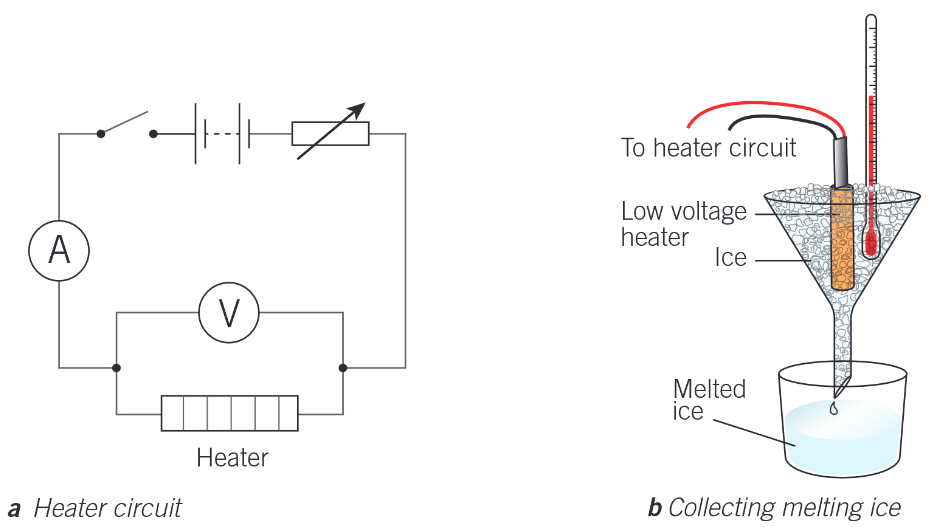
Describe the experiment to find specific latent heat of fusion
Method:
Setup equipment as shown in diagram. Place the beacker on a balance.
Read the thermometer and wait until the ice is at its melting point, not at a lower temperature. The the ice should be seen to be just starting to melt before the heater is switched on.
When the heater is turned on, record the mass of the beaker and any water in it using the balance
Meausure the potential difference V across the heater, the current I in the heater, and the time t during which the heater is used.
When the heater is switched off,
Analysis:
E = VIt and L = E / m, so Lf = VIt / m
State the zeroth law of thermodynamics
If two objects are each in thermal equilibrium with a third, then all three are in thermal equilibrium with each other.
What is the celsius scale?
A temperature scale with 100 degrees between the freezing point and the boiling point of pure water (at atmospheric pressure 1.01 x 103 Pa), 0°C and 100°C
What is the absolute temperature scale?
A scale for measuring temperature based on absolute zero and the triple point of pure water, with gradations equal in size to those of the Celsius scale; unit kelvin (K)