Synthetic Routes (copy)
5.0(1)
Card Sorting
1/16
Earn XP
Description and Tags
Last updated 5:35 PM on 3/27/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
1
New cards
Heat it under reflux using acidified potassium dichromate VI (K2Cr2O7/H2SO4) as an oxidising agent
How do you convert a primary alcohol into a carboxylic acid?
2
New cards
Distil it using acidified potassium dichromate VI (K2Cr2O7/H2SO4) as an oxidising agent
How do you convert a primary alcohol into an aldehyde?
3
New cards
Heat it under reflux using acidified potassium dichromate VI (K2Cr2O7/H2SO4) as an oxidising agent
How do you convert a secondary alcohol into a ketone?
4
New cards
Heat it under reflux using an acid catalyst (concentrated H2SO4/H3PO4)
How do you convert an alcohol into an alkene?
5
New cards
Heat under reflux with a sodium halide and sulfuric acid
How do you convert an alcohol into a haloalkane?
6
New cards
React with steam in the presence of an H3PO4 catalyst at 300C and 60-70 atm of pressure
How do you convert an alkene into an alcohol?
7
New cards
Heat under reflux with aqueous sodium hydroxide
How do you convert a haloalkane into an alcohol?
8
New cards
Nucleophilic substitution
What is the mechanism by which a haloalkane becomes an alcohol?
9
New cards

Draw the hydrolysis of bromoethane to become ethanol
10
New cards
React with a halogen at room temperature
How do you convert an alkene into a dihaloalkane?
11
New cards
React with a hydrogen halide at room temperature
How do you convert an alkene into a haloalkane?
12
New cards
Electrophilic addition
What is the mechanism by which an alkene becomes a (di)haloalkane?
13
New cards
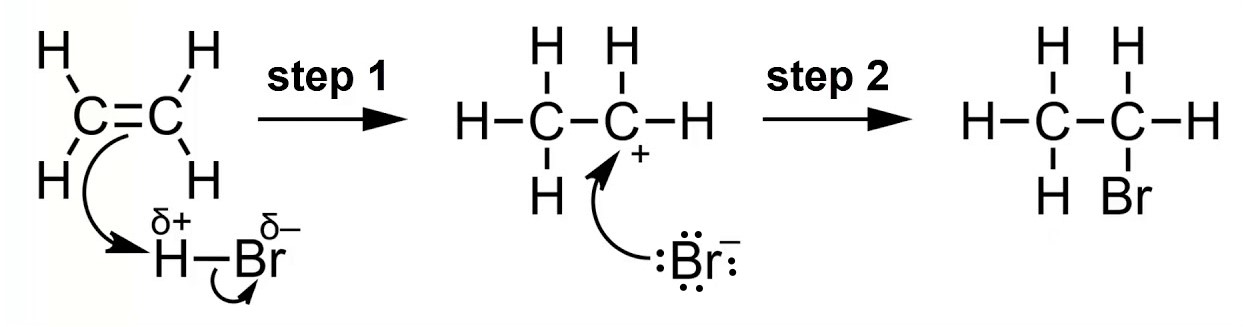
Draw the electrophilic addition of ethene with HBr to become bromoethane
14
New cards
React with hydrogen and pass over a nickel catalyst at 423K
How do you convert an alkene into an alkane?
15
New cards
React with a halogen under UV radiation
How do you convert an alkane into a haloalkane?
16
New cards
Radical substitution
What is the mechanism by which an alkane becomes a haloalkane?
17
New cards
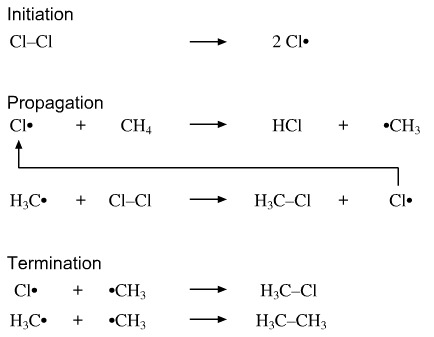
Draw the radical substitution of methane to become chloromethane