CHAPTER 4: MOLECULAR GEOMETRY, POLARITY, AND INTERMOLECULAR FORCES OF ATTRACTION CPQ
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
What is the molecular geometry of nitrogen trichloride (NCl3)?
trigonal pyramidal.
This table shows the relationship between the number of electron groups, the resulting electron geometries, and the corresponding angles between groups of electrons. Some of the blanks in the table have been filled in for you. The electron geometry in the cell labeled C in the table should be
# of Electron groups | Electron geometry | Angle between groups of electrons |
2 | linear | A |
3 | B | 120º |
4 | C | D |
tetrahedral.
Which statement BEST describes the difference between electron geometry and molecular geometry? |
Electron geometry is two dimensional, whereas molecule shapes are three dimensional.
Answers b., c., and d. are all true.
There is no real difference between these terms.
Electron geometry refers to the arrangement of electron groups around a central atom, whereas molecular geometry refers to the arrangement of atoms.
Electron geometry is theoretical, whereas molecular geometry is real.
Electron geometry refers to the arrangement of electron groups around a central atom, whereas molecular geometry refers to the arrangement of atoms.
This table shows the relationship between the number of electron groups, the resulting electron geometries, and the corresponding angles between groups of electrons. Some of the blanks in the table have been filled in for you. The angle in the cell labeled A in the table should be
# of Electron groups | Electron geometry | Angle between groups of electrons |
2 | linear | A |
3 | B | 120º |
4 | C | D |
180º
What is the angle between groups of electrons for an atom that has a linear electron geometry? |
180°
What is the electron geometry of the electron groups on the oxygen atom in methanol, shown below?
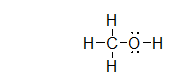
tetrahedral
What is the angle between groups of electrons for an atom that has a trigonal planar electron geometry?
120°
How many atoms are bonded to the carbon in dichloromethane (CH2Cl2)?
4
How many electron groups are found on the nitrogen atom of nitrogen trichloride (NCl3)?
4
This table shows the relationship between the number of electron groups, the resulting electron geometries, and the corresponding angles between groups of electrons. Some of the blanks in the table have been filled in for you. The electron geometry in the cell labeled B in the table should be
# of Electron groups | Electron geometry | Angle between groups of electrons |
2 | linear | A |
3 | B | 120º |
4 | C | D |
trigonal planar.
What is the electron geometry of the nitrogen atom in nitrogen trichloride (NCl3)?
tetrahedral
Why do electron groups around a central atom arrange themselves as far apart from one another as possible, while still remaining attached to the central atom?
The like charges of the electrons repel each other.
An atom in a molecule has one lone pair and three atoms bonded to it. What is the molecular geometry of this atom?
trigonal pyramidal
Which element is the LEAST electronegative? |
carbon
fluorine
oxygen
hydrogen
potassium
potassium
Nitrogen trichloride, once used as a bleaching agent, causes neurological disorder and was banned in 1949. Which of the following is a correct Lewis structure for nitrogen trichloride (NCl3)?
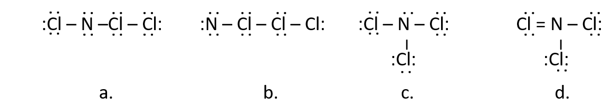
structure c |
Ethylene is used as a starting material in making plastics. It has a molecular formula of C2H4. Which of the following structures is the correct Lewis structure for ethylene?
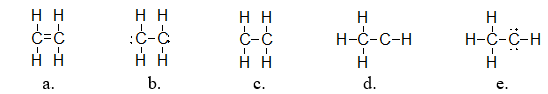
structure a
What is the electron geometry of each carbon in ethylene (C2H4)?
They are both trigonal planar.
What is the molecular geometry of each carbon in ethylene (C2H4)?
They are both trigonal planar.
What is the bond angle of the central atom in the given molecule?
109.5°
How many nonbonding pairs are on the carbon in dichloromethane (CH2Cl2)? |
0
Match the atomic symbol and ion symbol with the correct terms.
| Mg2+ | Cl- | Cδ+ | Fδ- |
a. | cation | anion | cation | anion |
b. | cation | anion | partially positive carbon | partially negative fluorine |
c. | anion | cation | anion | cation |
d. | cation | anion | partially negative carbon | partially positive fluorine |
e. | anion | cation | partially negative carbon | partially positive fluorine |
choice b |
Which element is the MOST electronegative? |
fluorine
chlorine
iodine
bromine
boron
fluorine
How does estradiol stimulate the growth of breast cancer cells?
Estradiol binds to breast cancer cell receptors as well as receptors found in other parts of the body.
An atom in a molecule has two lone pairs and two atoms bonded to it. What is the molecular geometry of this atom?
bent
Which element is the MOST electronegative?
selenium
carbon
oxygen
sulfur
nitrogen
oxygen
What is the electron geometry of the carbon in chloroform (CHCl3)?
tetrahedral
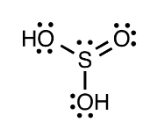
Determine the molecular geometry for the given compound:
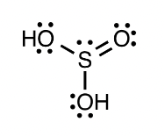
trigonal pyramidal
What is meant by the following symbols?
This means that the oxygen is pulling electrons toward itself and is partially negative. |
This table shows the relationship between the number of electron groups, the resulting electron geometries, and the corresponding angles between groups of electrons. Some of the blanks in the table have been filled in for you. The angle in the cell labeled D in the table should be
# of Electron groups | Electron geometry | Angle between groups of electrons |
2 | linear | A |
3 | B | 120º |
4 | C | D |
109.5º.
What is the electron geometry of SiO2?
linear
Which elements have the highest electronegativity and why? |
Nonmetals. They accept electrons in order to attain an octet.
Antiestrogens are one type of molecule that can be used to treat breast cancer. Which of the following characteristics should be included in the design of a novel antiestrogen?
The molecule should not have negative side effects.
All of the statements are design considerations.
The molecule should bind to the estrogen receptor on breast cancer cells.
The molecule should prevent the activation of genes in breast cancer cells.
The molecule should not interfere with the normal role of estrogen.
All of the statements are design considerations.
What is the molecular geometry of the carbon in formaldehyde, shown below?

trigonal planar
Chloroform (CHCl3) is an anesthetic and is also used in the synthesis of ozone-damaging refrigerants called CFCs. What is the correct Lewis dot structure for chloroform?
structure a
What is the angle between groups of electrons for an atom that has a tetrahedral electron geometry?
109.5° |
Electronegativity is a measure of an atom's ability to
draw electrons to itself in a covalent bond.
The structure below is a Lewis structure of methane. This Lewis structure tells us many things about methane that are useful. However, it also suggests one characteristic of methane that is, in fact, false. What is this one characteristic?
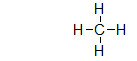
The Lewis structure suggests that methane is flat, but it really isn't.
What is the molecular geometry of carbon in chloroform (CHCl3)?
tetrahedral
When determining the shape of a molecule, it is necessary to count electron groups. Which of the following is an electron group?
a single bond
a triple bond
a nonbonding pair of electrons
a double bond
All of these are electron groups
All of these are electron groups.
What is the electron geometry of the carbon in formaldehyde, shown below?

trigonal planar |
What is the molecular geometry of the atoms bonded to oxygen in methanol, shown below?
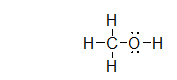
bent
How many electron groups do the carbon atoms in ethylene (C2H4) have? |
They both have three groups.