fatty acid synthesis + citrate-malate shuttle+ regulation (Slide deck 2)
1/21
Earn XP
Description and Tags
steps and structures
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
differences between fatty acid breakdown and biosynthesis
Occur by different pathways
Biosynthesis requires malonyl-CoA
Catalyzed by different sets of enzymes
Occur in different cellular compartments (in eukaryotes)
Breakdown occurs in the mitochondria
Biosynthesis occurs in the cytosol
fat synthesis: step 1
malonyl-CoA is formed from acetyl-CoA and bicarbonate
acetyl-CoA carboxylase = catalyzes the irreversible formation of malonyl-CoA from acetyl-CoA
Reaction occurs in the cytoplasm
Contains a biotin prosthetic group covalently bound in amide linkage to the 𝜀-amino group of a Lys residue
Step 1: the carboxyl group from HCO3- is transferred to biotin in an ATP-dependent reaction
The carboxyl group is carried by the biotin to a different active site, where the CO2 is transferred to acetyl-CoA to yield malonyl-CoA. This functions to make the subsequent steps more thermodynamically favorable.
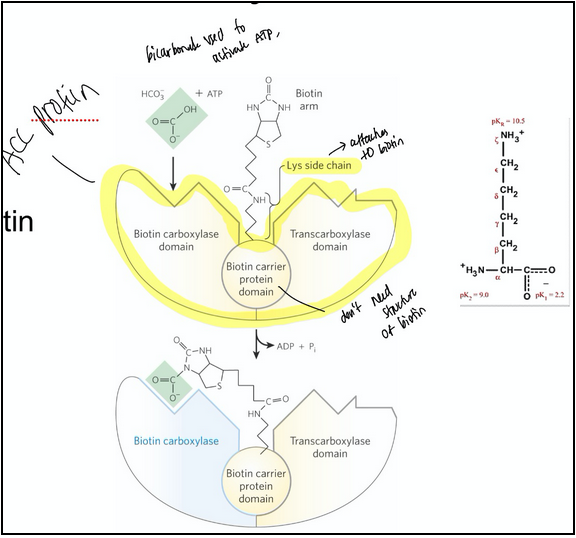
why is bicarbonate used to activate acetyl-CoA
It's a good leaving group once attached
It’s around → forms spontaneously when carbon dioxide dissolves in water
Biology is complex and doesn’t always use the most straightforward pathway
fatty acid synthesis: step 2
begins with malonyl-ACP and Acetyl group (from acetyl-CoA) at the KS domain
condensation reaction, CO2 lost allows it to be favorable
catalyzed by fatty acid synthase I
forming beta-ketoacyl-ACP
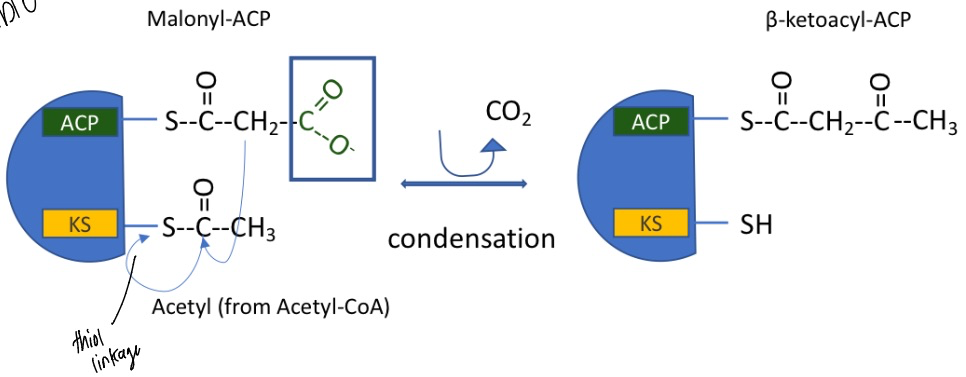
how many carbons does the chain elongate by?
2
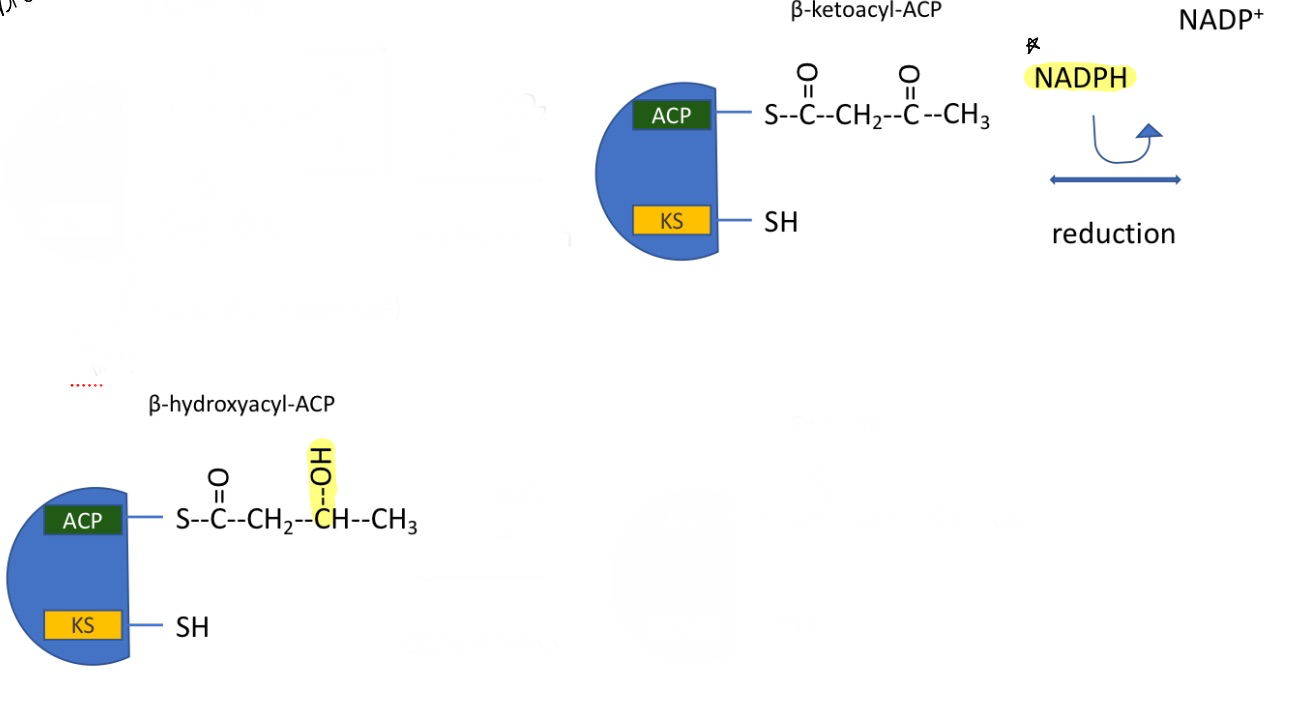
fatty acid synthesis: step 3
reduction reaction with NADPH in cytoplasm
doesn’t cost any ATP
catalyzed by fatty acid synthase I
forming beta-hydroxyacyl-ACP
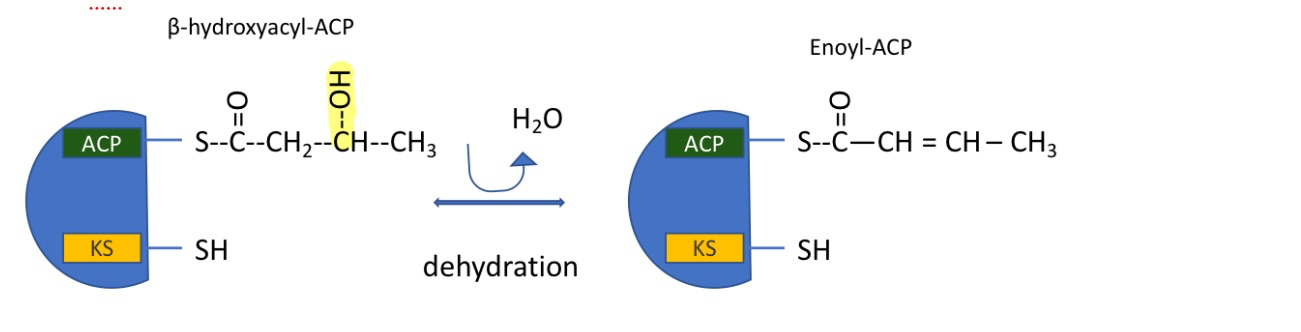
fatty acid synthesis: step 4
dehydration reaction which removes H2O
makes a double bond
catalyzed by fatty acid synthase I
forming enoyl-ACP
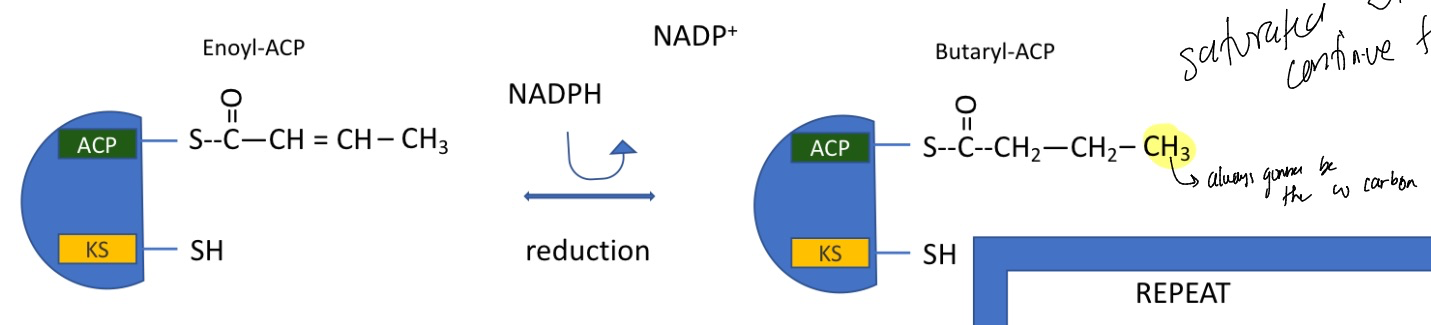
fatty acid synthesis: step 5
reduction reaction using NADPH
again doesn’t cost any energy
catalyzed by fatty acid synthase I
forms butaryl-ACP
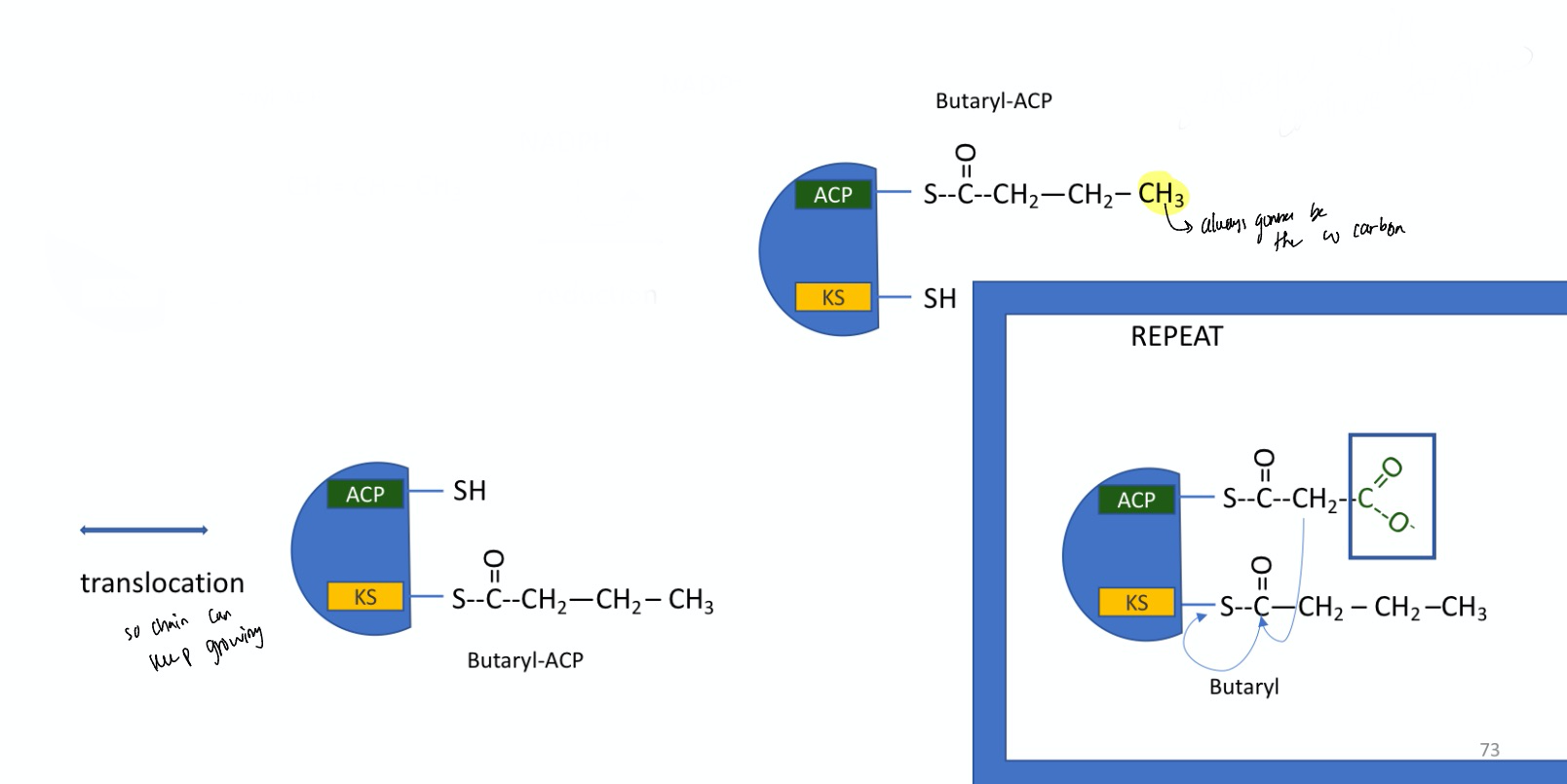
fatty acid synthesis: step 6
translocation step so chain can keep growing
rearranges and puts the thiol group on the ACP arm and the Butaryl on the KS domain
catalyzed by fatty acid synthase I
then the steps repeat to elongate chain again
a new incoming malonyl can be added until the FA is 16 carbons long
flexibility of ACP and KS domains
ACP arm is flexible but KS (β-ketoacyl synthase, where acetyl-CoA will bind, thiol linkage) is not flexible
difference between cofactor and activating groups in fatty acid breakdown and biosynthesis
In 𝛽 oxidation:
NAD and FAD serve as electron acceptors
The activating group is the thiol (-SH) group of coenzyme A
In fatty acid synthesis:
The reducing agent is NADPH
The activating groups are two different enzyme-bound -SH groups
fatty acid synthase 1 (FAS I) —> structure explanation
FAS I is found in mammals
Seven active sites are in separate domains within a single multifunctional polypeptide chain
The intermediates remain covalently attached as thioesters to one of two thiol groups:
The -SH group of a Cys residue in 𝛽-ketoacyl-ACP synthase (KS)
The -SH group of acyl carrier protein (ACP)
Two polypeptide chains function independently, but as a homodimer
Homodimer: The enzyme is made of two identical polypeptide chains (subunits) that come together to form the functional complex
One chain can carry out fatty acid synthesis on its own → that’s what “function independently” means.
Two of these chains naturally dimerize (stick together in a head-to-tail orientation).
As a homodimer, they work more efficiently — the growing fatty acid chain can even “swing” between the two monomers during synthesis.
ACP arm within protein can go to reaction centers since it is flexible
how do substrates within the fatty acid synthase I know where to go?
Substrates know which site to go to based on attraction and lock and key
Each catalytic domain has a specific binding pocket with the right shape and chemical attractions
The flexible ACP arm increases efficiency because it can reach multiple domains, but the chemical recognition ensures it doesn’t “dock” at the wrong place.
why is the dimer organization of fatty acid synthase I imporant?
The dimeric organization makes the process more tolerant of mistakes, because there’s a “backup” active site and the intermediates can switch over
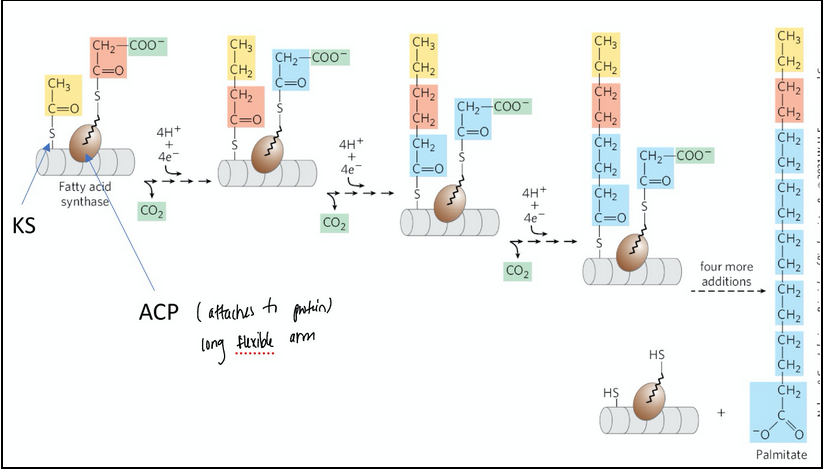
The overall process of palmitate synthesis ** might have carbon tracking question
Carbons C-16 and C-15 of the palmitate are derived from the methyl and carboxyl carbon atoms, respectively, of an acetyl-CoA used to prime the system at the outset
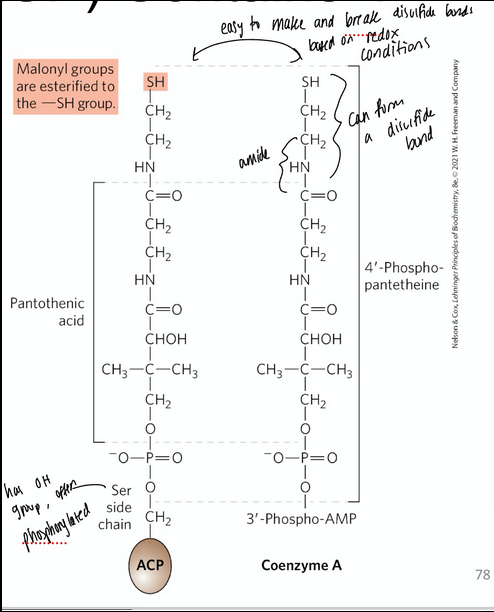
Acyl carrier protein (ACP)
Contains 4’-phosphopantetheine
4’-phosphopantetheine = a prosthetic group of ACP that serves as a flexible arm
Also in coenzyme A
Tethers the fatty acyl chain to the surface of the FAS complex
Carries reaction intermediates from one active site to the next
Acyl carrier protein (ACP) and coenzyme A
Incredibly similar structure
And are great leaving groups
The acyl carrier protein (ACP) itself is not a leaving group; instead, it carries an acyl group attached to its phosphopantetheine (PPT) arm via a thioester bond
biosynthesis of fatty acid requires (3)
Acetyl-CoA
The group transfer potential of ATP to make malonyl-CoA
The reducing power of NADPH to reduce the 𝛽-keto group and the double bond
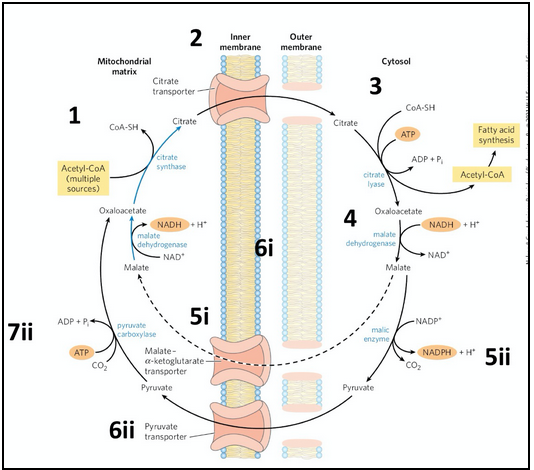
citrate-malate shuttle
We need to get the acetyl-CoA which is required to make a lipid into the cytoplasm
Acetyl-CoA is produced in the mitochondrial matrix by pyruvate dehydrogenase -- but acetyl-CoA cannot diffuse through the mitochondrial membrane to the cytoplasm
TCA/Kreb’s cycle converts acetyl-CoA into citrate and there IS a transporter for citrate
Citrate in the cytoplasm is converted into acetyl-CoA by citrate lyase → for fatty acid synthesis
But now need to restore the mitochondrial stores of citrate
The product of citrate lyase is oxaloacetate, which after conversion to malate, can diffuse through the outer mitochondrial membrane and be transported back into the matrix
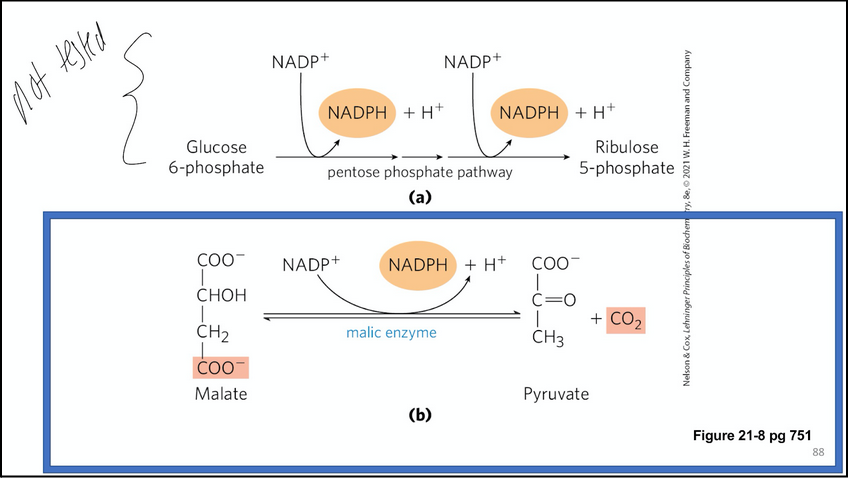
OR: malate can be converted to pyruvate by malic enzyme which is transported into the matrix by the pyruvate transporter and converted into oxaloacetate. This pathway involving malic enzyme produces NADPH -- which is required for fatty acid syntehsis and is one of the major ways in which the cell generates this essential electron acceptor.
The return of oxaloacetate back into the mitochdonrial matrix is facilitated by one of these two transporters:
Malate-𝛼-ketogluterate transporter = transports malate into the matrix where it is reoxidized to oxaloacetate by malate dehydrogenase
Pyruvate transporter = transports pyruvate into the matrix where it is converted to oxaloacetate by pyruvate carboxylase
two main ways of generating cytosolic NADPH
pentose phosphate pathway
malic enzyme (citrate-malate shuttle)
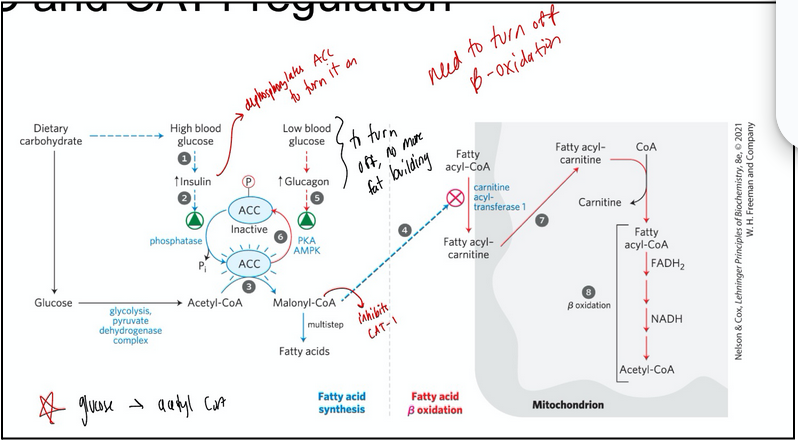
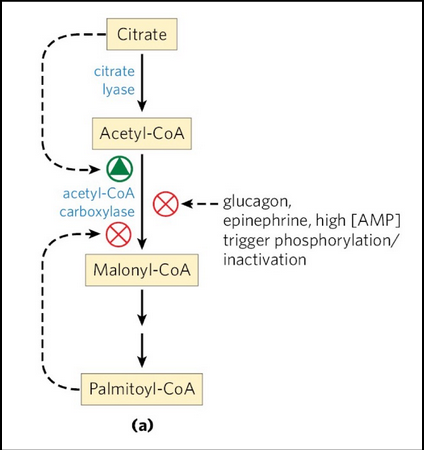
acetyl-CoA carboxylase (ACC) regulation
We want to synthesize fatty acids when there is an abundance of energy and acetyl-CoA available
We want to reduce/restrict synthesis when there is not
acetyl-CoA carboxylase -- which synthesizes malonyl-CoA (a required substrate for FA synthesis) is inhibited by glucagon (secreted when blood glucose levels are low in the fasting state) and by high levels of palmitoyl-CoA the ultimate product of fatty acid synthase (FAS)
Negatively regulated by phosphorylation
ACC and CAT1 regulation
ACC -- which makes malonyl-CoA is inhibited when phosphorylated
High blood glucose dephosphorylates this enzyme making it more active
The product, malonyl-CoA inhibits carnitine acyl-transferase I. Restricting the amount og fatty acyl-CoA that can enter the mitochondria for oxidative breakdown
In the fasting state ACC is inactive and malonyl CoA is not being synthesized and beta-oxidation is favoured.